Salicylhydrazide

Salicylhydrazide structure
|
Common Name | Salicylhydrazide | ||
|---|---|---|---|---|
| CAS Number | 936-02-7 | Molecular Weight | 152.15100 | |
| Density | 1.318g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C7H8N2O2 | Melting Point | 147-150 °C(lit.) | |
| MSDS | N/A | Flash Point | N/A | |
| Name | Salicylhydrazide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.318g/cm3 |
|---|---|
| Melting Point | 147-150 °C(lit.) |
| Molecular Formula | C7H8N2O2 |
| Molecular Weight | 152.15100 |
| Exact Mass | 152.05900 |
| PSA | 75.35000 |
| LogP | 1.08690 |
| Index of Refraction | 1.622 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi:Irritant; |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| WGK Germany | 3 |
| RTECS | VO3700000 |
| HS Code | 2928000090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Synthesis, Characterization, and Anticancer Activity of New Metal Complexes Derived from 2-Hydroxy-3-(hydroxyimino)-4-oxopentan-2-ylidene)benzohydrazide.
Bioinorg. Chem. Appl. 2015 , 126023, (2015) Novel metal(II) complexes derived from 2-hydroxy-N'-((Z)-3-(hydroxyimino)-4-oxopentan-2-ylidene)benzohydrazide ligand (H2L) were synthesized and characterized by elemental and thermal analyses (DTA an... |
|
|
Salicylidene salicylhydrazide, a selective inhibitor of beta 1-containing GABAA receptors.
Br. J. Pharmacol. 142 , 97-106 , (2004) 1. A high-throughput assay utilizing the voltage/ion probe reader (VIPR) technology identified salicylidene salicylhydrazide (SCS) as being a potent selective inhibitor of alpha2beta1gamma1 GABA(A) re... |
|
|
Discovery of novel non-cytotoxic salicylhydrazide containing HIV-1 integrase inhibitors.
Bioorg. Med. Chem. Lett. 17(23) , 6472-5, (2007) The previously discovered salicylhydrazide class of compounds displayed potent HIV-1 integrase (IN) inhibitory activity. The development of this class of compounds as antiretroviral agents was halted ... |
| MFCD00007599 |
| 2-hydroxybenzohydrazide |
| EINECS 213-311-3 |
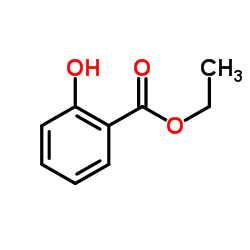 CAS#:118-61-6
CAS#:118-61-6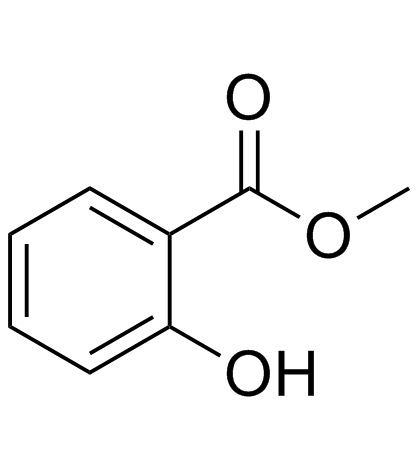 CAS#:119-36-8
CAS#:119-36-8 CAS#:69-72-7
CAS#:69-72-7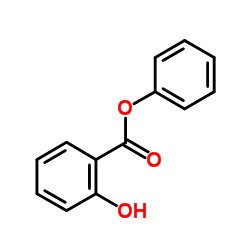 CAS#:118-55-8
CAS#:118-55-8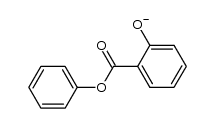 CAS#:61141-14-8
CAS#:61141-14-8 CAS#:59395-02-7
CAS#:59395-02-7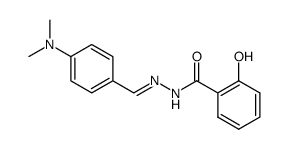 CAS#:87444-18-6
CAS#:87444-18-6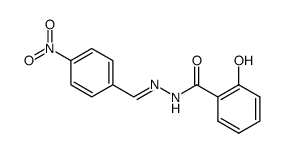 CAS#:72323-41-2
CAS#:72323-41-2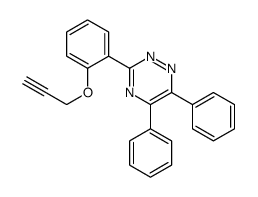 CAS#:106823-25-0
CAS#:106823-25-0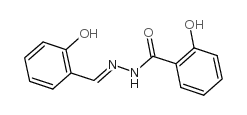 CAS#:3232-36-8
CAS#:3232-36-8 CAS#:90-02-8
CAS#:90-02-8![(2S,5R,6R)-6-[[2-[[(2E)-2-[(2-hydroxybenzoyl)hydrazinylidene]acetyl]amino]-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid structure](https://image.chemsrc.com/caspic/492/143667-43-0.png) CAS#:143667-43-0
CAS#:143667-43-0 CAS#:51362-49-3
CAS#:51362-49-3 CAS#:938-73-8
CAS#:938-73-8 CAS#:20349-50-2
CAS#:20349-50-2 CAS#:18176-38-0
CAS#:18176-38-0 CAS#:20602-57-7
CAS#:20602-57-7![Benzoic acid,2-hydroxy-, 2-[(2-methoxyphenyl)methylene]hydrazide structure](https://image.chemsrc.com/caspic/244/18176-35-7.png) CAS#:18176-35-7
CAS#:18176-35-7
