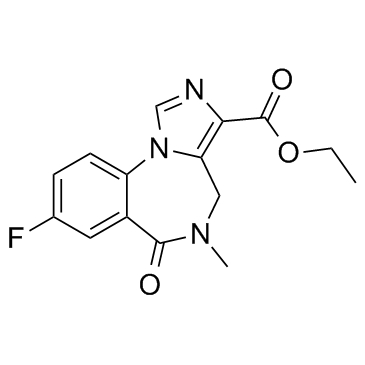
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
NI2922170
-
CHEMICAL NAME :
-
4H-Imidazo(1,5-a)(1,4)benzodiazepine-3-carboxylic acid, 8-fluoro-5,6-dihydro-5-methyl-6- oxo-, ethyl ester
-
CAS REGISTRY NUMBER :
-
78755-81-4
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
15
-
MOLECULAR FORMULA :
-
C15-H14-F-N3-O3
-
MOLECULAR WEIGHT :
-
303.32
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
5700 ng/kg/C
-
TOXIC EFFECTS :
-
Cardiac - cardiomyopathy including infarction Cardiac - arrhythmias (including changes in conduction) Cardiac - change in rate
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 304,1415,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
2 mg/kg/2D-I
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - excitement Behavioral - aggression
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 339,488,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
4 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Cardiac - EKG changes not diagnostic of specified effects Cardiac - pulse rate increase, without fall in BP
-
REFERENCE :
-
PECAE5 Pediatric Emergency Care. (Williams & Wilkins, 428 E. Preston St., Baltimore, MD 21202) V.1- 1985- Volume(issue)/page/year: 11,186,1995
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4200 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - tremor Behavioral - rigidity (including catalepsy)
-
REFERENCE :
-
JTCEEM Journal de Toxicologie Clinique et Experimentale. (SPPIF, B.P.22, F-41353 Vineuil, France) V.5- 1985- Volume(issue)/page/year: 7,223,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1360 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DRFUD4 Drugs of the Future. (J.R. Prous, S.A., Apartado de Correos 540, 08080 Barcelona, Spain) V.1- 1975/76- Volume(issue)/page/year: 7,402,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
85 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Behavioral - rigidity (including catalepsy)
-
REFERENCE :
-
JTCEEM Journal de Toxicologie Clinique et Experimentale. (SPPIF, B.P.22, F-41353 Vineuil, France) V.5- 1985- Volume(issue)/page/year: 7,223,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1300 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - tremor Behavioral - rigidity (including catalepsy)
-
REFERENCE :
-
JTCEEM Journal de Toxicologie Clinique et Experimentale. (SPPIF, B.P.22, F-41353 Vineuil, France) V.5- 1985- Volume(issue)/page/year: 7,223,1987
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DRFUD4 Drugs of the Future. (J.R. Prous, S.A., Apartado de Correos 540, 08080 Barcelona, Spain) V.1- 1975/76- Volume(issue)/page/year: 7,402,1982
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,201,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
143 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Behavioral - rigidity (including catalepsy)
-
REFERENCE :
-
JTCEEM Journal de Toxicologie Clinique et Experimentale. (SPPIF, B.P.22, F-41353 Vineuil, France) V.5- 1985- Volume(issue)/page/year: 7,223,1987
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>640 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,201,1992
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>30 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 23,201,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
2 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - tremor Behavioral - rigidity (including catalepsy)
-
REFERENCE :
-
JTCEEM Journal de Toxicologie Clinique et Experimentale. (SPPIF, B.P.22, F-41353 Vineuil, France) V.5- 1985- Volume(issue)/page/year: 7,223,1987 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
21 mg/kg
-
SEX/DURATION :
-
female 14-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - behavioral
-
REFERENCE :
-
PNPPD7 Progress in Neuro-Psychopharmacology and Biological Psychiatry. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.6- 1982- Volume(issue)/page/year: 17,151,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
220 mg/kg
-
SEX/DURATION :
-
female 11-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - behavioral
-
REFERENCE :
-
GEPHDP General Pharmacology. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.6- 1975- Volume(issue)/page/year: 22,43,1991
|
 CAS#:428507-28-2
CAS#:428507-28-2 CAS#:193693-31-1
CAS#:193693-31-1 CAS#:999-97-3
CAS#:999-97-3 CAS#:99-97-8
CAS#:99-97-8 CAS#:3400-09-7
CAS#:3400-09-7 CAS#:2999-46-4
CAS#:2999-46-4 CAS#:865-47-4
CAS#:865-47-4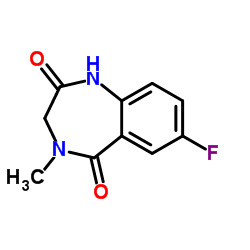 CAS#:78755-80-3
CAS#:78755-80-3![8-amino-5,6-dihydro-5-methyl-6-oxo-4H-imidazol[1,5-a][1,4]benzodiazepine-3-carboxylic acid ethyl ester Structure](https://image.chemsrc.com/caspic/482/658075-93-5.png) CAS#:658075-93-5
CAS#:658075-93-5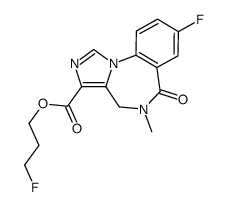 CAS#:133368-73-7
CAS#:133368-73-7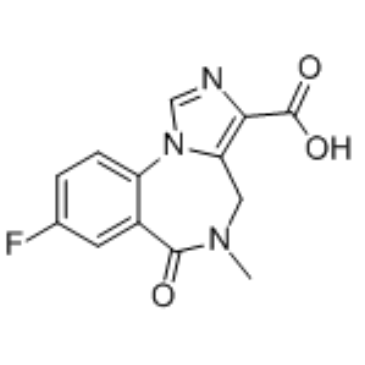 CAS#:84378-44-9
CAS#:84378-44-9