beta-Estradiol 17-hemisuccinate
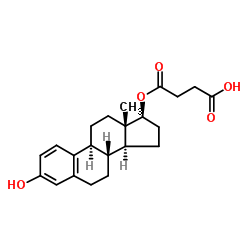
beta-Estradiol 17-hemisuccinate structure
|
Common Name | beta-Estradiol 17-hemisuccinate | ||
|---|---|---|---|---|
| CAS Number | 7698-93-3 | Molecular Weight | 372.455 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 570.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H28O5 | Melting Point | 162-164°C (lit.) | |
| MSDS | Chinese USA | Flash Point | 198.7±23.6 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
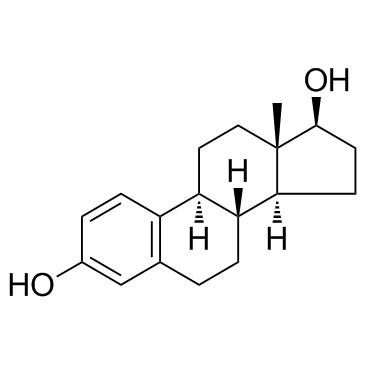
Use of beta-Estradiol 17-hemisuccinateβ-Estradiol 17-hemisuccinate is a synthetic derivative of estradiol (HY-B0141)[1]. |
| Name | 4-[[(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]oxy]-4-oxobutanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | β-Estradiol 17-hemisuccinate is a synthetic derivative of estradiol (HY-B0141)[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 570.1±50.0 °C at 760 mmHg |
| Melting Point | 162-164°C (lit.) |
| Molecular Formula | C22H28O5 |
| Molecular Weight | 372.455 |
| Flash Point | 198.7±23.6 °C |
| Exact Mass | 372.193665 |
| PSA | 83.83000 |
| LogP | 4.64 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.598 |
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H332-H351 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | 20/21/22-40 |
| Safety Phrases | 22-36 |
| RIDADR | NONH for all modes of transport |
|
~94% 
beta-Estradiol ... CAS#:7698-93-3 |
| Literature: Zhao, Ming; Liu, Jiangyuan; Zhang, Xiaoyi; Peng, Li; Li, Chunyu; Peng, Shiqi Bioorganic and Medicinal Chemistry, 2009 , vol. 17, # 10 p. 3680 - 3689 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats.
J. Neuroimmunol. , (2008) Estrogen has been shown to attenuate the inflammatory response following injury or lipopolysaccharide treatment in several organ systems. Estrogen's actions are transduced through two estrogen recepto... |
|
|
Steroid-bovine serum albumin conjugates: molecular characterization and their interaction with androgen and estrogen receptors.
J. Steroid Biochem. 24(5) , 1017-31, (1986) Conjugates of testosterone-3-carboxymethyloxime (T-3-CMO), testosterone-17-hemisuccinate (T-17-HS), 17 beta-estradiol-6-carboxymethyloxime (E-6-CMO), or 17 beta-estradiol-17-hemisuccinate (E-17-HS) an... |
|
|
Prevention of bridge binding in immunoassays: a general estradiol tracer structure.
J. Steroid Biochem. 32(2) , 251-7, (1989) Iodinated estradiol tracers were synthesized with three different bridges connecting the radiolabelled moiety to the steroid core: Hemisuccinate, carboxymethyloxime and amide. Taking these iodinated t... |
| 17beta-Estradiol hemisuccinate |
| 1,3,5(10)-Estratriene-3,17beta-diol 17-hemisuccinate |
| 4-{[(14beta,17alpha)-3-Hydroxyestra-1,3,5(10)-Trien-17-Yl]oxy}-4-Oxobutanoic Acid |
| Eutocol (TN) |
| 4-{[(17β)-3-Hydroxyestra-1,3,5(10)-trien-17-yl]oxy}-4-oxobutanoic acid |
| 17BETA-ESTRADIOL 17-HEMISUCCINATE |
| Estradiol-17-hemisuccinate |
| Butanedioic acid, mono[(17β)-3-hydroxyestra-1,3,5(10)-trien-17-yl] ester |
| beta-Estradiol 17-hemisuccinate |