CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
VB2002000
-
CHEMICAL NAME :
-
3-Quinolinecarboxylic acid, 1,4-dihydro-1-ethyl-6-fluoro-7-(4-methyl-1-piperaziny l)-4-oxo-
-
CAS REGISTRY NUMBER :
-
70458-92-3
-
BEILSTEIN REFERENCE NO. :
-
0567618
-
LAST UPDATED :
-
199806
-
DATA ITEMS CITED :
-
9
-
MOLECULAR FORMULA :
-
C17-H20-F-N3-O3
-
MOLECULAR WEIGHT :
-
333.40
-
WISWESSER LINE NOTATION :
-
T66 BN VVJ B2 DVQ HF I- AT6N DNTJ D1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
1714 mg/kg/21W-I
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - paresthesis Peripheral Nerve and Sensation - structural change in nerve or sheath
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
343 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Musculoskeletal - joints Musculoskeletal - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
16 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - muscle contraction or spasticity
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
225 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>50 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
7500 mg/kg/30D-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - other changes in urine composition Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
30 gm/kg
-
SEX/DURATION :
-
male 30 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
MUTATION DATA
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
-
TEST SYSTEM :
-
Rodent - rat Liver
-
DOSE/DURATION :
-
780 umol/L
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 221,263,1989
|
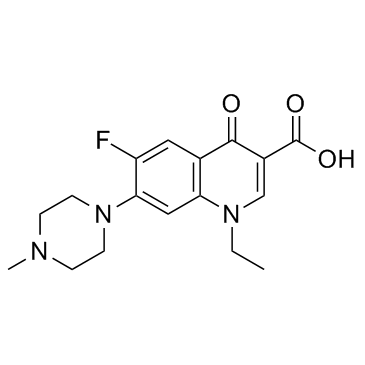
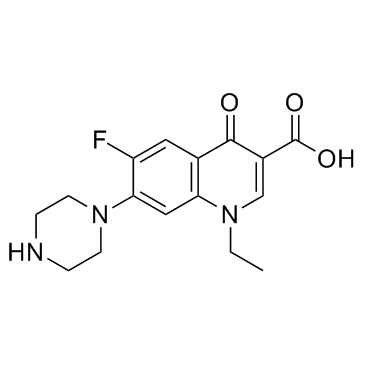 CAS#:70458-96-7
CAS#:70458-96-7 CAS#:74-88-4
CAS#:74-88-4 CAS#:50-00-0
CAS#:50-00-0 CAS#:109-01-3
CAS#:109-01-3 CAS#:68077-26-9
CAS#:68077-26-9 CAS#:7440-44-0
CAS#:7440-44-0 CAS#:74-83-9
CAS#:74-83-9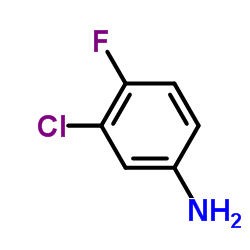 CAS#:367-21-5
CAS#:367-21-5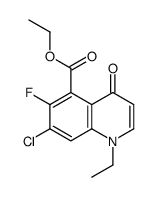 CAS#:70458-94-5
CAS#:70458-94-5![diethyl [[(3-chloro-4-fluorophenyl)amino]methylene]malonate Structure](https://image.chemsrc.com/caspic/354/70032-30-3.png) CAS#:70032-30-3
CAS#:70032-30-3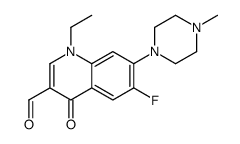 CAS#:110719-58-9
CAS#:110719-58-9