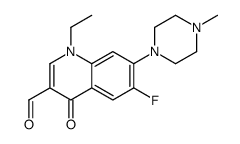
70458-92-3
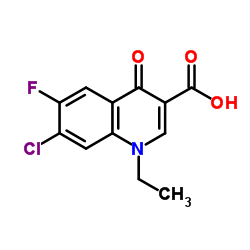
| Name | 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid |
|---|---|
| Synonyms |
MFCD01685696
PFLX Abactal Pefloxacine Labocton Silver Pefloxacin Pefloxacin 1-Ethyl-6-fluoro-7-(4-methyl-1-piperazinyl)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid Pefloxacino 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid Pefloxacin (USAN) Pefloxacinum EINECS 274-611-8 |
| Description | Pefloxacin is a an antibacterial agent and prevents bacterial DNA replication by inhibiting DNA gyrase (topoisomerse)Target: DNA gyrasePefloxacin is a synthetic chemotherapeutic agent used to treat severe and life-threatening bacterial infections. Pefloxacin is commonly referred to as afluoroquinolone (or quinolone) drug and is a member of the fluoroquinolone class of antibacterials. It is an analog of norfloxacin. It is a synthetic fluoroquinolone, belonging to the 3rd generation of quinolones. Pefloxacin is extensively prescribed in France. Pefloxacin has not been approved for use in the United States.The bactericidal action of pefloxacin results from interference with the activity of the bacterial enzymes DNA gyrase and topoisomerase IV, which are needed for the transcription and replication of bacterial DNA. DNA gyrase appears to be the primary quinolone target for gram-negative bacteria. Topoisomerase IV appears to be the preferential target in gram-positive organisms. Interference with these two topoisomerases results in strand breakage of the bacterial chromosome, supercoiling, and resealing. As a result DNA replication and transcription is inhibited. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 529.1±50.0 °C at 760 mmHg |
| Molecular Formula | C17H20FN3O3 |
| Molecular Weight | 333.357 |
| Flash Point | 273.8±30.1 °C |
| Exact Mass | 333.148865 |
| PSA | 65.78000 |
| LogP | 1.51 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.594 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | N |
|---|---|
| Risk Phrases | R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . |
| Safety Phrases | S61 |
| RIDADR | UN 3082 |
| RTECS | WM6250000 |
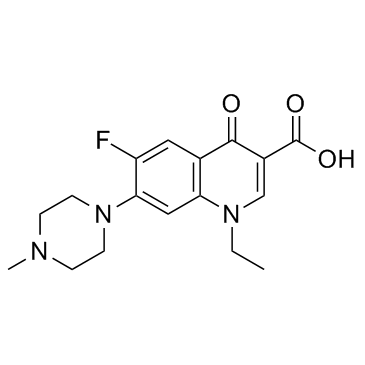
| Precursor 10 | |
|---|---|
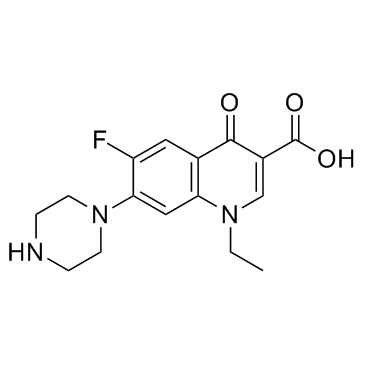
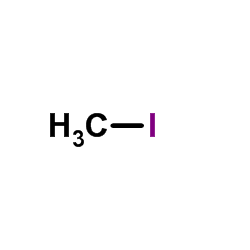
| DownStream 1 | |

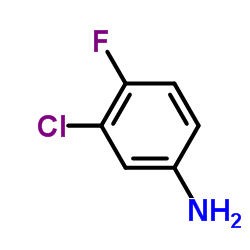
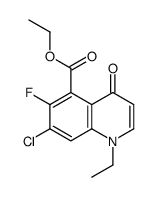
![diethyl [[(3-chloro-4-fluorophenyl)amino]methylene]malonate structure](https://image.chemsrc.com/caspic/354/70032-30-3.png)