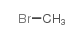70458-92-3
| 中文名 | 培氟沙星 |
|---|---|
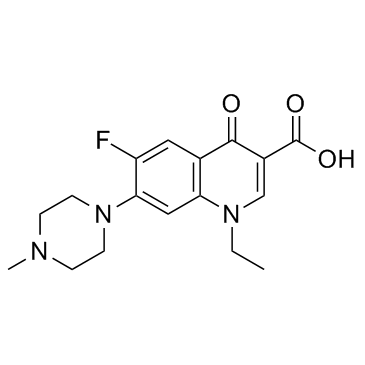
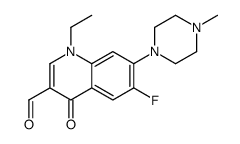
| 英文名 | 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid |
| 中文别名 |
1-乙基-6-氟-1,4-二氢-7-(4-佳绩-哌嗪基)-4-氧代-3-喹啉羧酸
培氟哌酸 1-乙基-1,4-二氢-6-氟-7-(4-甲基-1-哌嗪)-4-氧代-3-喹啉羧酸 1-乙基-6-氟-7-(4-甲基哌嗪基)-4-氧代-1,4-二氢喹啉-3-羧酸 |
| 英文别名 |
MFCD01685696
PFLX Abactal Pefloxacine Labocton Silver Pefloxacin Pefloxacin 1-Ethyl-6-fluoro-7-(4-methyl-1-piperazinyl)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid Pefloxacino 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid Pefloxacin (USAN) Pefloxacinum EINECS 274-611-8 |
| 描述 | Pefloxacin是抗菌化合物,通过抑制DNA旋转酶来阻断DNA复制。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 529.1±50.0 °C at 760 mmHg |
| 分子式 | C17H20FN3O3 |
| 分子量 | 333.357 |
| 闪点 | 273.8±30.1 °C |
| 精确质量 | 333.148865 |
| PSA | 65.78000 |
| LogP | 1.51 |
| 蒸汽压 | 0.0±1.5 mmHg at 25°C |
| 折射率 | 1.594 |
| 储存条件 | 2-8℃ |
| 分子结构 | 1、 摩尔折射率:85.63 2、 摩尔体积(cm3/mol):252.4 3、 等张比容(90.2K):679.3 4、 表面张力(dyne/cm):52.4 5、 极化率(10-24cm3):33.94 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:1 3.氢键受体数量:7 4.可旋转化学键数量:3 5.互变异构体数量:无 6.拓扑分子极性表面积64.1 7.重原子数量:24 8.表面电荷:0 9.复杂度:545 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.性状:类白色晶体。 2.熔点(ºC):270--272 3.溶解性:溶于碱性和酸性溶液,微溶于水。 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| 危害码 (欧洲) | N |
|---|---|
| 风险声明 (欧洲) | R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . |
| 安全声明 (欧洲) | S61 |
| 危险品运输编码 | UN 3082 |
| RTECS号 | WM6250000 |
| 上游产品 10 | |
|---|---|
| 下游产品 1 | |