Erythromycin stearate
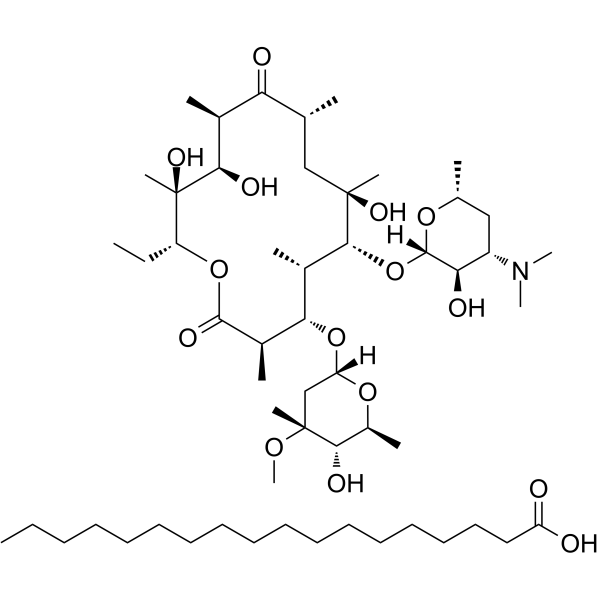
Erythromycin stearate structure
|
Common Name | Erythromycin stearate | ||
|---|---|---|---|---|
| CAS Number | 643-22-1 | Molecular Weight | 1004.377 | |
| Density | 1.112g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C55H103NO15 | Melting Point | 77-79ºC | |
| MSDS | Chinese USA | Flash Point | 523.101ºC | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of Erythromycin stearateErythromycin stearate is a macrolide antibiotic produced by actinomycete Streptomyces erythreus with a broad spectrum of antimicrobial activity. Erythromycin stearate binds to bacterial 50S ribosomal subunits and inhibits RNA-dependent protein synthesis by blockage of transpeptidation and/or translocation reactions, without affecting synthesis of nucleic acid[1][2]. Erythromycin stearate also exhibits antitumor and neuroprotective effect in different fields of research[3][4]. |
| Name | Erythromycin Stearate |
|---|---|
| Synonym | More Synonyms |
| Description | Erythromycin stearate is a macrolide antibiotic produced by actinomycete Streptomyces erythreus with a broad spectrum of antimicrobial activity. Erythromycin stearate binds to bacterial 50S ribosomal subunits and inhibits RNA-dependent protein synthesis by blockage of transpeptidation and/or translocation reactions, without affecting synthesis of nucleic acid[1][2]. Erythromycin stearate also exhibits antitumor and neuroprotective effect in different fields of research[3][4]. |
|---|---|
| Related Catalog | |
| Target |
Bacterial; RNA-dependent protein synthesis[1] |
| In Vitro | Erythromycin stearate inhibits growth of P. falciparum with IC50 and IC90 values of 58.2 μM and 104.0 μM, respectively[1]. Erythromycin stearate (10 μM, 100 μM; 24 h, 72 h) shows antioxidant and anti-inflammatory effects and suppresses the accumulation of 4-HNE (p<0.01) and 8-OHdG (p<0.01), reduces Iba-1 (p<0.01) and TNF-α (p<0.01) expression significantly[4]. Cell Viability Assay[4] Cell Line: Embryos primary cortical neuron (from the cerebral cortices of 17-day-old Sprague-Dawley rat) Concentration: 10, 100 μM Incubation Time: 24, 72 hours Result: Improved the viability of cultured neuronal cells in vitro after 3 hours oxygen-glucose deprivation (OGD). |
| In Vivo | Erythromycin stearate (gastric intubation; 0.1-50 mg/kg; 30-120 days) decreases tumor growth and prolong the survival time of mice from dose of 5 mg/kg in mice[3]. Erythromycin stearate (gastric intubation; 5 mg/kg) protects mice alive even at 120 days after inoculation, but shortens mean survival time in tumor-bearing mice by 4-5 days with dose of 50 mg/kg[3]. Erythromycin stearate (i.h.; single injection; 50 mg/kg) has a protective effect on the rat model with cerebral ischemia reperfusion-injury[4]. Animal Model: Female ddY mice (6-week-old) with EAC cells or CDF mice (6-week-old) with P388 cells[3] Dosage: 0.1 mg/kg; 0.5 mg/kg; 10 mg/kg; 30 mg/kg; 50 mg/kg Administration: Gastric intubation; 30-120 days Result: Decreased tumor growth and prolonged the mean survival time of mice from the dose of 5 mg/kg, however, the 50 mg/kg dosage shortened the MST in tumorbearing mice. Animal Model: Male Sprague-Dawley rats (8-week-old, 250-300 g)[4] Dosage: 50 mg/kg Administration: Subcutaneous single injection Result: Reduced infarct volume and edema volume, improved neurological deficit. |
| References |
[1]. Gribble MJ, et al. Erythromycin. Med Clin North Am. 1982 Jan;66(1):79-89. [3]. K Hamada, et al. Antitumor Effect of Erythromycin in Mice. Chemotherapy |
| Density | 1.112g/cm3 |
|---|---|
| Melting Point | 77-79ºC |
| Molecular Formula | C55H103NO15 |
| Molecular Weight | 1004.377 |
| Flash Point | 523.101ºC |
| Exact Mass | 1003.717102 |
| PSA | 231.21000 |
| LogP | 8.11810 |
| Index of Refraction | 1.518 |
| InChIKey | YAVZHCFFUATPRK-YZPBMOCRSA-N |
| SMILES | CCC1OC(=O)C(C)C(OC2CC(C)(OC)C(O)C(C)O2)C(C)C(OC2OC(C)CC(N(C)C)C2O)C(C)(O)CC(C)C(=O)C(C)C(O)C1(C)O.CCCCCCCCCCCCCCCCCC(=O)O |
| Storage condition | 2-8°C |
| Stability | Stable. Combustible. Incompatible with acids, strong oxidizing agents. |
| Water Solubility | ethanol: soluble50mg/mL, clear to very slightly hazy, colorless |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H317-H334 |
| Precautionary Statements | P261-P280-P284-P304 + P340-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R42/43 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | KF5785000 |
| HS Code | 2942000000 |
| HS Code | 2942000000 |
|---|
|
Antibiotics for ureaplasma in the vagina in pregnancy.
Cochrane Database Syst. Rev. (9) , CD003767, (2011) Preterm birth is a significant perinatal problem contributing to perinatal morbidity and mortality. Heavy vaginal ureaplasma colonisation is suspected of playing a role in preterm birth and preterm ru... |
|
|
Cholestasis and liver cell damage due to hypersensitivity to erythromycin stearate--recurrence following therapy with erythromycin succinate.
Wien. Klin. Wochenschr. 111(2) , 76-7, (1999) Erythromycin is a frequently used antibiotic in patients with atypical respiratory infection and/or an allergy to penicillin. We report the case of a young woman who developed severe cholestasis and j... |
|
|
Efficacy and tolerance of roxithromycin in comparison with erythromycin stearate in patients with lower respiratory tract infections.
Scand. J. Infect. Dis. 24(2) , 219-25, (1992) The efficacy and tolerance of roxithromycin 150 mg b.i.d. were compared with those of erythromycin stearate 500 mg b.i.d. in patients with lower respiratory tract infections. Out of 86 patients recrui... |
| EINECS 211-396-1 |
| MFCD00084690 |
| Heptadecanoic acid - (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-{[(2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl]oxy}-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione (1:1) |
| Erythromycin stearate |

