1,3,5-Trimethoxybenzene
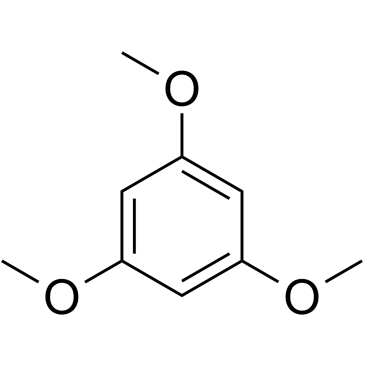
1,3,5-Trimethoxybenzene structure
|
Common Name | 1,3,5-Trimethoxybenzene | ||
|---|---|---|---|---|
| CAS Number | 621-23-8 | Molecular Weight | 168.190 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 257.0±20.0 °C at 760 mmHg | |
| Molecular Formula | C9H12O3 | Melting Point | 50-53 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 85.6±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 1,3,5-Trimethoxybenzene1,3,5-Trimethoxybenzene is a key component of the Chinese rose odor. 1,3,5-Trimethoxybenzene is synthesized in three successive methylation steps from phloroglucinol, the initial precursor. 1,3,5-Trimethoxybenzene is an effective sedative[1]. |
| Name | 1,3,5-trimethoxybenzene |
|---|---|
| Synonym | More Synonyms |
| Description | 1,3,5-Trimethoxybenzene is a key component of the Chinese rose odor. 1,3,5-Trimethoxybenzene is synthesized in three successive methylation steps from phloroglucinol, the initial precursor. 1,3,5-Trimethoxybenzene is an effective sedative[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 257.0±20.0 °C at 760 mmHg |
| Melting Point | 50-53 °C(lit.) |
| Molecular Formula | C9H12O3 |
| Molecular Weight | 168.190 |
| Flash Point | 85.6±0.0 °C |
| Exact Mass | 168.078644 |
| PSA | 27.69000 |
| LogP | 1.61 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.485 |
| InChIKey | LKUDPHPHKOZXCD-UHFFFAOYSA-N |
| SMILES | COc1cc(OC)cc(OC)c1 |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S24/25 |
| RIDADR | 1325 |
| WGK Germany | 3 |
| RTECS | DC2810000 |
| Packaging Group | III |
| Hazard Class | 4.1 |
| HS Code | 29093090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2909309090 |
|---|---|
| Summary | 2909309090 other aromatic ethers and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
A denuder-impinger system with in situ derivatization followed by gas chromatography-mass spectrometry for the determination of gaseous iodine-containing halogen species.
J. Chromatogr. A. 1210(2) , 135-41, (2008) Reactive iodine species have been suggested to play an important role in the atmosphere (e.g. tropospheric ozone depletion, coastal new particle formation). However, there still exist major uncertaint... |
|
|
Ozonolysis of lignin models in aqueous solution: anisole, 1,2-dimethoxybenzene, 1,4-dimethoxybenzene, and 1,3,5-trimethoxybenzene.
Environ. Sci. Technol. 43(16) , 6275-82, (2009) The lignin models anisole, 1,2-dimethoxybenzene, 1,4-dimethoxybenzene, and 1,3,5-trimethoxybenzene were reacted with ozone in aqueous solution, and major products were identified and quantified with r... |
|
|
Stereoselective glycosylations using oxathiane spiroketal glycosyl donors.
Carbohydr. Res. 348 , 6-13, (2012) Novel oxathiane spiroketal donors have been synthesised and activated via an umpolung S-arylation strategy using 1,3,5-trimethoxybenzene and 1,3-dimethoxybenzene. The comparative reactivity of the res... |
| Benzene,1,3,5-trimethoxy |
| 1,3,5-trimethoxy-benzene |
| Benzene, 1,3,5-trimethoxy- |
| 1,3,5-Trimethoxybenzene |
| tri-O-methylphloroglucinol |
| phloroglucinol trimethyl ether |
| 2,4,6-Trimethoxybenzene |
| 1,3,5-Trimethyoxybenzene |
| 1,3,5-trimethoxy-benzen |
| sym-Trimethoxybenzene |
| 2,4,6-trimethoxyiodobenzene |
| 1,3,5-Trimethoxybenz |
| 1,3,5-trimethoxyarene |
| MFCD00008385 |
| 5-TriMethoxybenzene |
| TRISMETHOXYBENZENE |
| 3,5-DIMETHOXYANISOLE |
| EINECS 210-673-4 |
 CAS#:626-39-1
CAS#:626-39-1 CAS#:108-73-6
CAS#:108-73-6 CAS#:77-78-1
CAS#:77-78-1 CAS#:570-02-5
CAS#:570-02-5 CAS#:100-39-0
CAS#:100-39-0 CAS#:135159-25-0
CAS#:135159-25-0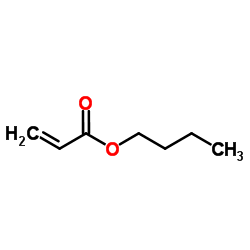 CAS#:141-32-2
CAS#:141-32-2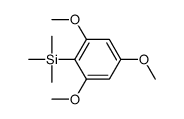 CAS#:36086-05-2
CAS#:36086-05-2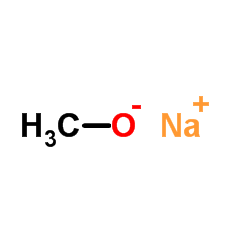 CAS#:124-41-4
CAS#:124-41-4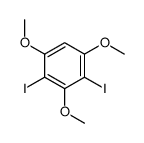 CAS#:117934-81-3
CAS#:117934-81-3 CAS#:3420-72-2
CAS#:3420-72-2 CAS#:491-70-3
CAS#:491-70-3 CAS#:14262-07-8
CAS#:14262-07-8 CAS#:1219118-19-0
CAS#:1219118-19-0 CAS#:15222-53-4
CAS#:15222-53-4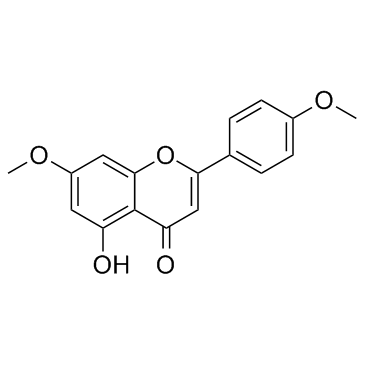 CAS#:5128-44-9
CAS#:5128-44-9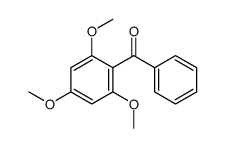 CAS#:3770-80-7
CAS#:3770-80-7 CAS#:493-77-6
CAS#:493-77-6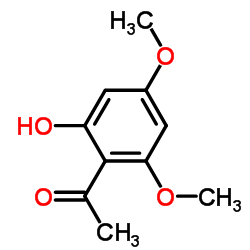 CAS#:90-24-4
CAS#:90-24-4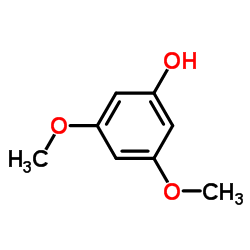 CAS#:500-99-2
CAS#:500-99-2
