alpha-Mangostin
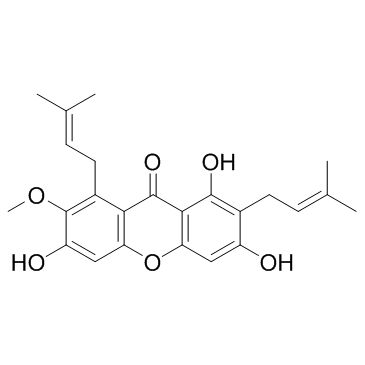
alpha-Mangostin structure
|
Common Name | alpha-Mangostin | ||
|---|---|---|---|---|
| CAS Number | 6147-11-1 | Molecular Weight | 410.460 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 640.1±55.0 °C at 760 mmHg | |
| Molecular Formula | C24H26O6 | Melting Point | 182ºC | |
| MSDS | Chinese USA | Flash Point | 220.3±25.0 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of alpha-MangostinAlpha-mangostin is a dietary xanthone with broad biological activities, such as antioxidant, anti-allergic, antiviral, antibacterial, anti-inflammatory and anticancer effects. It is an inhibitor of mutant IDH1 (IDH1-R132H) with a Ki of 2.85 μM. |
| Name | α-mangostin |
|---|---|
| Synonym | More Synonyms |
| Description | Alpha-mangostin is a dietary xanthone with broad biological activities, such as antioxidant, anti-allergic, antiviral, antibacterial, anti-inflammatory and anticancer effects. It is an inhibitor of mutant IDH1 (IDH1-R132H) with a Ki of 2.85 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2.85 μM (IDH1-R132H)[1] |
| In Vitro | Alpha-mangostin exhibits a selective inhibitory effect on IDH1-R132H, but not on IDH1. Alpha-mangostin competitively inhibits the binding of alpha-mangostin (α-KG) to IDH1-R132H. The structure–relationship study reveals that alpha-mangostin exhibits the strongest core inhibitor structure. Alpha-mangostin selectively promotes demethylation of 5-methylcytosine (5mC) and histone H3 trimethylated lysine residues in IDH1 (+/R132H) MCF10A cells[1]. Cell proliferation significantly decreases in a dose-dependent manner in the cells treated with alpha-mangostin. Alpha-mangostin also increases the levels of Bax (pro-apoptotic), cleaved caspase-3, cleaved caspase-9 and cleaved-poly(ADP-ribose) polymerase (PARP)[2]. Alpha-mangostin significantly inhibits light-induced degeneration of photoreceptors and 200 μM H2O2-induced apoptosis of RPE cells. 200 μM H2O2-induced generation of reactive oxygen species (ROS) and light-induced generation of malondialdehyde (MDA) are suppressed by alpha-mangostin[3]. |
| In Vivo | Alpha-mangostin reduces risk of liver fibrosis through the decrease in p53 expression as compared to the TAA_DMSO treatment. The serum levels of the liver enzymes AST and ALT after treatment with α-mangostin decrease as compared to DMSO alone[4]. |
| Cell Assay | IDH1+/+ and IDH1 MCF10A cells are grown in DMEM/F-12 media, supplemented with 5% horse serum, 20 ng/mL EGF, 0.5 μg/mL hydrocortisone, 10 μg/mL insulin. IDH1+/+ and IDH1 MCF10A cells are seeded in 6 well plates. After an exposure to 5 μM alpha-mangostin. cells are collected after indicated times and the viable cell number is calculated, using hemacytometer counting[1]. |
| Animal Admin | Rats: Male Wistar rats are divided into 3 groups and treated with intraperitoneal injections of TAA (200 mg/kg). One subgroup is left untreated whereas the other two are treated either with 100 mg/kg alpha-mangostin or vehicle alone (80% DMSO, 20% water), which are administered intraperitoneally 3 times per weekfor a total of4 weeks. The incidence offibrotic nodules on the liver and the serum levels of the liver enzymes aspartate transaminase (AST) and alanine transaminase (ALT) are measured[4]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 640.1±55.0 °C at 760 mmHg |
| Melting Point | 182ºC |
| Molecular Formula | C24H26O6 |
| Molecular Weight | 410.460 |
| Flash Point | 220.3±25.0 °C |
| Exact Mass | 410.172943 |
| PSA | 100.13000 |
| LogP | 5.45 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.624 |
| InChIKey | GNRIZKKCNOBBMO-UHFFFAOYSA-N |
| SMILES | COc1c(O)cc2oc3cc(O)c(CC=C(C)C)c(O)c3c(=O)c2c1CC=C(C)C |
| Storage condition | 2-8°C |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Alpha-mangostin induces changes in glutathione levels associated with glutathione peroxidase activity in rat brain synaptosomes.
Nutr. Neurosci. 15(5) , 13-9, (2012) In a previous report, we have characterized the antiperoxidative properties of alpha-mangostin in different toxic models tested in nerve tissue preparations.Here, the modulatory effects of this xantho... |
|
|
A miniaturized flow-through cell to evaluate skin permeation of endoxifen.
Int. J. Pharm. 441(1-2) , 433-40, (2013) Endoxifen, an anti-estrogenic agent, has been recently implicated in the use of breast cancer. Its physicochemical properties make it a good candidate for transdermal delivery. However, as an investig... |
|
|
Neuroprotective effect of α-mangostin and curcumin against iodoacetate-induced cell death.
Nutr. Neurosci. 15(5) , 34-41, (2012) Curcumin is a phenolic yellow curry pigment with anti-inflammatory and antioxidant activities and α-mangostin is a xanthone isolated from mangosteen fruit with antioxidant properties. Iodoacetate (IAA... |
| 1,3,6-Trihydroxy-7-methoxy-2,8-di(3-methyl-2-butenyl)xanthone |
| Mangostin |
| 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-enyl)xanthen-9-one |
| alpha-mangostin |
| EINECS 448-420-7 |
| a-Mangostin |
| 1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)-9H-xanthen-9-one |
| 9H-Xanthen-9-one, 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-buten-1-yl)- |
| 1,3,6-Trihydroxy-7-méthoxy-2,8-bis(3-méthyl-2-butèn-1-yl)-9H-xanthén-9-one |
| 1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methyl-2-buten-1-yl)-9H-xanthen-9-one |
| 9H-Xanthen-9-one, 1,3,6-trihydroxy-7-methoxy-2,8-bis(3-methyl-2-butenyl)- |
| 1,3,6-Trihydroxy-7-methoxy-2,8-bis(3-methylbut-2-en-1-yl)-9H-xanthen-9-one |
| MFCD00135200 |
 CAS#:426820-49-7
CAS#:426820-49-7 CAS#:108-73-6
CAS#:108-73-6![2-[3-(1,3-benzodioxol-5-yl)-4-oxoquinazolin-2-yl]sulfanyl-N-ethyl-N-(3-methylphenyl)acetamide Structure](https://image.chemsrc.com/caspic/389/6195-80-8.png) CAS#:6195-80-8
CAS#:6195-80-8 CAS#:13246-46-3
CAS#:13246-46-3 CAS#:120677-47-6
CAS#:120677-47-6 CAS#:95-01-2
CAS#:95-01-2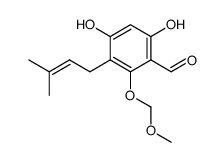 CAS#:426820-54-4
CAS#:426820-54-4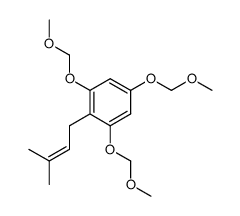 CAS#:426820-42-0
CAS#:426820-42-0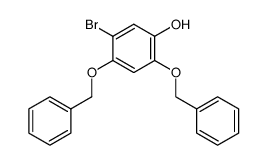 CAS#:426820-51-1
CAS#:426820-51-1 CAS#:426820-39-5
CAS#:426820-39-5![5,9-Dihydroxy-8-methoxy-2,2-dimethyl-7-(3-methyl-2-buten-1-yl)-3,4-dihydro-2H,6H-pyrano[3,2-b]xanthen-6-one structure](https://image.chemsrc.com/caspic/218/19275-46-8.png) CAS#:19275-46-8
CAS#:19275-46-8 CAS#:19275-44-6
CAS#:19275-44-6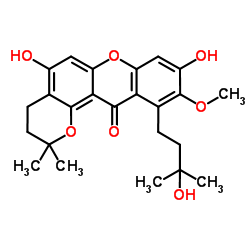 CAS#:26063-96-7
CAS#:26063-96-7![3,4-dihydro-5,9-dihydroxy-8-methoxy-7-(3-methoxy-3-methylbutyl)-2,2-dimethyl-2H,6H-pyrano-[3,2-b]xanthen-6-one structure](https://image.chemsrc.com/caspic/259/112649-47-5.png) CAS#:112649-47-5
CAS#:112649-47-5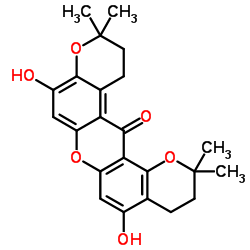 CAS#:15404-80-5
CAS#:15404-80-5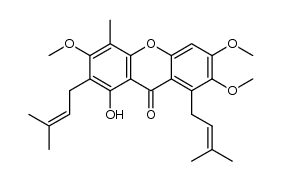 CAS#:1285705-91-0
CAS#:1285705-91-0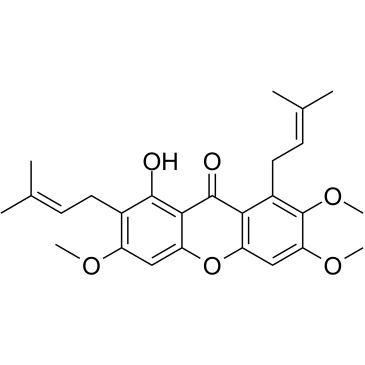 CAS#:15404-76-9
CAS#:15404-76-9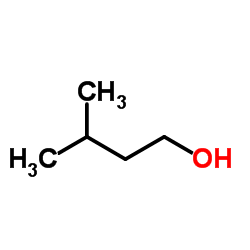 CAS#:123-51-3
CAS#:123-51-3 CAS#:4630-06-2
CAS#:4630-06-2
