Glycopyrrolate
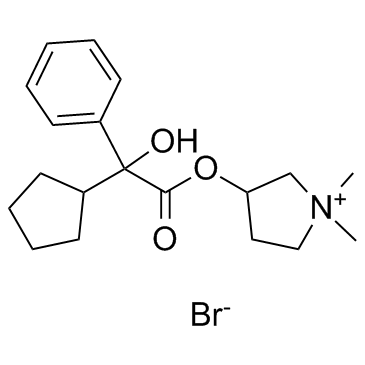
Glycopyrrolate structure
|
Common Name | Glycopyrrolate | ||
|---|---|---|---|---|
| CAS Number | 596-51-0 | Molecular Weight | 398.335 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C19H28BrNO3 | Melting Point | 193 - 194.5ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of GlycopyrrolateGlycopyrrolate(Glycopyrronium Br) is a muscarinic competitive antagonist used as an antispasmodic.IC50 Value:Target: mAChR (Muscarinic acetylcholine receptor M1)in vitro: Glycopyrrolate showed no selectivity in its binding to the M1-M3 receptors. Kinetics studies, however, showed that glycopyrrolate dissociates slowly from HASM muscarinic receptors (60% protection against [3H]-NMS binding at 30 nM) compared to ipratropium bromide [1].in vivo: Glycopyrrolate (1 mg) tablets were then administered, starting with one tablet daily the third week and increasing the daily dose by one tablet per week until a maximum of four tablets during week six and 4 days of week seven when the daily dose was reduced to two tablets for 3 days. glycopyrrolate can be given in controlled doses provided that an adequate medical assessment has been undertaken [2]. Glycopyrrolate has a slow and erratic absorption from the gastrointestinal system, but even low plasma levels are associated with a distinct and long-lasting antisialogic effect [3]. Oral glycopyrrolate is emerging as a potential second-line treatment option, but experience with safety, efficacy, and dosing is especially limited in children [4]. phase III study, 52.3% of glycopyrrolate oral solution recipients (aged 3-18 years; n = 137) had an mTDS response (primary endpoint); the response rate was consistently above 50% at all 4-weekly timepoints, aside from the first assessment at week 4 (40.3%). In general, glycopyrrolate oral solution was well tolerated in clinical trials. The majority of adverse events were within expectations as characteristic anticholinergic outcomes [5].Toxicity: Side effects include dry mouth, difficult urinating, heachaches, diarrhea and constipation. The medication also induces drowsiness or blurred vision. LD50=709 mg/kg (rat, oral). |
| Name | Glycopyrrolate Bromide |
|---|---|
| Synonym | More Synonyms |
| Description | Glycopyrrolate(Glycopyrronium Br) is a muscarinic competitive antagonist used as an antispasmodic.IC50 Value:Target: mAChR (Muscarinic acetylcholine receptor M1)in vitro: Glycopyrrolate showed no selectivity in its binding to the M1-M3 receptors. Kinetics studies, however, showed that glycopyrrolate dissociates slowly from HASM muscarinic receptors (60% protection against [3H]-NMS binding at 30 nM) compared to ipratropium bromide [1].in vivo: Glycopyrrolate (1 mg) tablets were then administered, starting with one tablet daily the third week and increasing the daily dose by one tablet per week until a maximum of four tablets during week six and 4 days of week seven when the daily dose was reduced to two tablets for 3 days. glycopyrrolate can be given in controlled doses provided that an adequate medical assessment has been undertaken [2]. Glycopyrrolate has a slow and erratic absorption from the gastrointestinal system, but even low plasma levels are associated with a distinct and long-lasting antisialogic effect [3]. Oral glycopyrrolate is emerging as a potential second-line treatment option, but experience with safety, efficacy, and dosing is especially limited in children [4]. phase III study, 52.3% of glycopyrrolate oral solution recipients (aged 3-18 years; n = 137) had an mTDS response (primary endpoint); the response rate was consistently above 50% at all 4-weekly timepoints, aside from the first assessment at week 4 (40.3%). In general, glycopyrrolate oral solution was well tolerated in clinical trials. The majority of adverse events were within expectations as characteristic anticholinergic outcomes [5].Toxicity: Side effects include dry mouth, difficult urinating, heachaches, diarrhea and constipation. The medication also induces drowsiness or blurred vision. LD50=709 mg/kg (rat, oral). |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 193 - 194.5ºC |
|---|---|
| Molecular Formula | C19H28BrNO3 |
| Molecular Weight | 398.335 |
| Exact Mass | 397.125244 |
| PSA | 46.53000 |
| Storage condition | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Distribution and density of contacts from noradrenergic and serotonergic boutons on the dendrites of neck flexor motoneurons in the adult cat.
J. Comp. Neurol. 523 , 1701-16, (2015) Serotonergic (5-HT) and noradrenergic (NA) input to spinal motoneurons is essential for generating plateau potentials and self-sustained discharges. Extensor motoneurons are densely innervated by 5-HT... |
|
|
Low birth weight male guinea pig offspring display increased visceral adiposity in early adulthood.
PLoS ONE 9(6) , e98433, (2014) Uteroplacental insufficiency (UPI)-induced intrauterine growth restriction (IUGR) predisposes individuals to adult visceral obesity. We postulated that low birth weight (LBW) offspring, from UPI-induc... |
|
|
Assessment of sensorimotor gating following selective lesions of cholinergic pedunculopontine neurons.
Eur. J. Neurosci. 40(10) , 3526-37, (2014) Sensorimotor gating is the state-dependent transfer of sensory information into a motor system. When this occurs at an early stage of the processing stream it enables stimuli to be filtered out or par... |
| Pyrrolidinium, 3-((cyclopentylhydroxyphenylacetyl)oxy)-1,1-dimethyl-, bromide |
| Pyrrolidinium, 3-[(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethyl-, bromide (1:1) |
| Tarodyl |
| Glycopyrrolate |
| MFCD00072137 |
| 3-Hydroxy-1,1-dimethylpyrrolidinium bromide α-cyclopentylmandelate |
| glycopyrronium bromide |
| TARODYN |
| Robanul |
| 3-{[cyclopentyl(hydroxy)phenylacetyl]oxy}-1,1-dimethylpyrrolidinium bromide |
| EINECS 209-887-0 |
| 3-[2-Cyclopentyl(hydroxy)phenylacetoxy]-1,1-dimethylpyrrolidinium bromide |
| pyrrolidinium, 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl-, bromide |
| Nodapton |
| Robinul |

