Prunetin
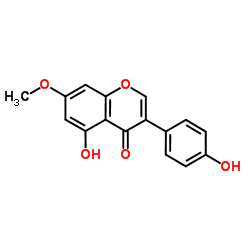
Prunetin structure
|
Common Name | Prunetin | ||
|---|---|---|---|---|
| CAS Number | 552-59-0 | Molecular Weight | 284.263 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 546.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H12O5 | Melting Point | 240-242ºC | |
| MSDS | Chinese USA | Flash Point | 209.7±23.6 °C | |
Use of PrunetinPrunetin, an O-methylated isoflavone, possesses anti-inflammatory activity. Prunetin is a potent human aldehyde dehydrogenases inhibitor[1][2]. |
| Name | prunetin |
|---|---|
| Synonym | More Synonyms |
| Description | Prunetin, an O-methylated isoflavone, possesses anti-inflammatory activity. Prunetin is a potent human aldehyde dehydrogenases inhibitor[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Prunetin inhibited LPS-induced inflammatory cytokine production and MUC5 AC expression and secretion by inactivating the TLR4/MyD88 pathway in human nasal epithelial cells[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 546.5±50.0 °C at 760 mmHg |
| Melting Point | 240-242ºC |
| Molecular Formula | C16H12O5 |
| Molecular Weight | 284.263 |
| Flash Point | 209.7±23.6 °C |
| Exact Mass | 284.068481 |
| PSA | 79.90000 |
| LogP | 3.53 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.669 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DJ3100050 |
| HS Code | 2914509090 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Preparation and evaluation of surface-bonded tricationic ionic liquid silica as stationary phases for high-performance liquid chromatography.
J. Chromatogr. A. 1396 , 62-71, (2015) Two tricationic ionic liquids were prepared and then bonded onto the surface of supporting silica materials through "thiol-ene" click chemistry as new stationary phases for high-performance liquid chr... |
|
|
Absorption of red clover isoflavones in human subjects: results from a pilot study.
Br. J. Nutr. 103(11) , 1569-72, (2010) In addition to soya-derived preparations, red clover-based dietary supplements have gained considerable interest as an alternative isoflavone (IF) source. While metabolism and bioavailability of the m... |
|
|
The phytoestrogen prunetin affects body composition and improves fitness and lifespan in male Drosophila melanogaster.
FASEB J. 30 , 948-58, (2016) Dietary isoflavones, a group of secondary plant compounds that exhibit phytoestrogenic properties, are primarily found in soy. Prunetin, a representative isoflavone, was recently found to affect cell ... |
| 5-Hydroxy-3-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one |
| Prunusetin |
| 5,4'-dihydroxy-7-methoxyisoflavone |
| Prunetin |
| 7-methoxy-4',5-dihydroxyisoflavone |
| 5-hydroxy-3-(4-hydroxyphenyl)-7-methoxychromen-4-one |
| 7-O-methyl-genistein |
| 4',5-dihydroxy-7-methoxyisoflavone |
| 4',5-dihydroxy-7-methoxygenistein |
| 4H-1-Benzopyran-4-one, 5-hydroxy-3-(4-hydroxyphenyl)-7-methoxy- |
| EINECS 209-018-5 |
| MFCD00016951 |
 CAS#:446-72-0
CAS#:446-72-0 CAS#:74-88-4
CAS#:74-88-4 CAS#:69127-80-6
CAS#:69127-80-6 CAS#:89595-66-4
CAS#:89595-66-4 CAS#:1162-82-9
CAS#:1162-82-9 CAS#:69127-79-3
CAS#:69127-79-3 CAS#:855829-21-9
CAS#:855829-21-9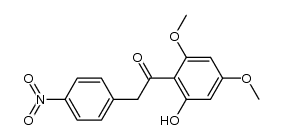 CAS#:56982-36-6
CAS#:56982-36-6 CAS#:108-73-6
CAS#:108-73-6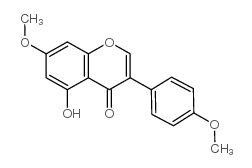 CAS#:34086-51-6
CAS#:34086-51-6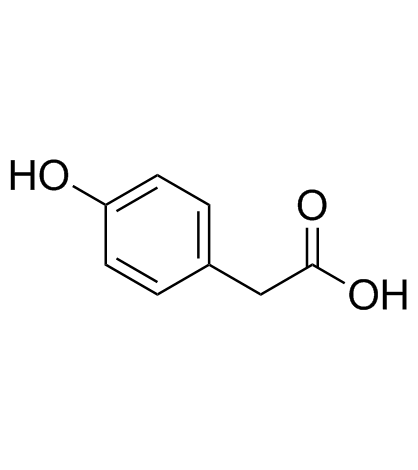 CAS#:156-38-7
CAS#:156-38-7
