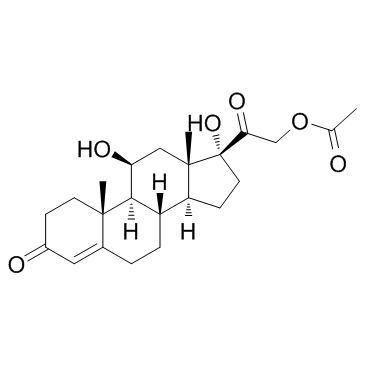
Prednisolone Acetate
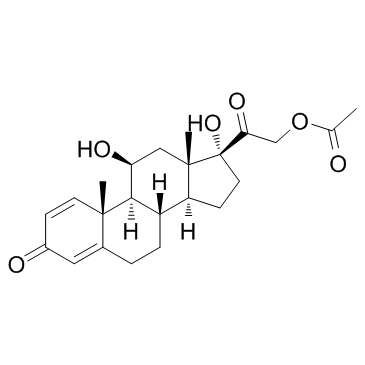
Prednisolone Acetate structure
|
Common Name | Prednisolone Acetate | ||
|---|---|---|---|---|
| CAS Number | 52-21-1 | Molecular Weight | 402.481 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 579.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C23H30O6 | Melting Point | 240-244 °C | |
| MSDS | Chinese USA | Flash Point | 198.4±23.6 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Prednisolone AcetatePrednisolone 21-acetate is an adrenal cortico hormones, with anti-inflammatory, anti-allergic and immune suppressive effects. |
| Name | Prednisolone Acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Prednisolone 21-acetate is an adrenal cortico hormones, with anti-inflammatory, anti-allergic and immune suppressive effects. |
|---|---|
| Related Catalog |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 579.8±50.0 °C at 760 mmHg |
| Melting Point | 240-244 °C |
| Molecular Formula | C23H30O6 |
| Molecular Weight | 402.481 |
| Flash Point | 198.4±23.6 °C |
| Exact Mass | 402.204254 |
| PSA | 100.90000 |
| LogP | 2.58 |
| Vapour Pressure | 0.0±3.7 mmHg at 25°C |
| Index of Refraction | 1.587 |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
|
Section 1. Chemical Product and Company Identification Prednisolone acetateCatalog YY1423, P1219, PR100 Common Name/ Number(s). Trade Name CAS#52-21-1
Manufacturer RTECSTU4152500 SPECTRUM CHEMICAL MFG. CORP. TSCATSCA 8(b) inventory: Prednisolone acetate Commercial Name(s)Prednisolone 21-Acetate; Cormalone; Cortpred; Meticortelone CI#Not available. acetate; Nisolone; Prediacortine; Perdicort (11beta)-11,17,21-Trihydroxypregna-1,4-diene-3,20-dione acetate Synonym IN CASE OF EMERGENCY Chemical Name (11.beta.)- Chemical FamilyNot available.CALL (310) 516-8000 Chemical FormulaC21H26O5 SPECTRUM CHEMICAL MFG. CORP. Section 2.Composition and Information on Ingredients Exposure Limits TWA (mg/m3)STEL (mg/m3) CEIL (mg/m3) NameCAS #% by Weight 1) Prednisolone acetate52-21-1100 Not applicable. Toxicological Data on Ingredients Section 3. Hazards Identification Potential Acute Health Effects Slightly hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation. CARCINOGENIC EFFECTS: Not available. Potential Chronic Health EffectsMUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Classified Reproductive system/toxin/female, Reproductive system/toxin/male [POSSIBLE]. Repeated or prolonged exposure is not known to aggravate medical condition. Prednisolone acetate Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionThese products are carbon oxides (CO, CO2). Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. of Various Substances SMALL FIRE: Use DRY chemical powder. Fire Fighting Media and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Not available. Special Remarks on Fire Hazards Special Remarks on Explosion Not available. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Prednisolone acetate Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not breathe dust. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal Protection Safety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Solid crystalline powder.)OdorOdorless. TasteNot available. Molecular Weight402.49 g/mole ColorWhite. pH (1% soln/water)Not available. Boiling PointNot available. Melting PointDecomposes. (237°C or 458.6°F) Critical TemperatureNot available. Not available. Specific Gravity Vapor PressureNot applicable. Vapor DensityNot available. VolatilityNot available. Odor ThresholdNot available. Not available. Water/Oil Dist. Coeff. Ionicity (in Water)Not available. See solubility in water, methanol, acetone. Dispersion Properties SolubilitySoluble in methanol, acetone. Section 10. Stability and Reactivity Data StabilityThe product is stable. Not available. Instability Temperature Conditions of InstabilityNot available. Incompatibility with variousNot available. substances CorrosivityNon-corrosive in presence of glass. Prednisolone acetate Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity PolymerizationNo. Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Toxicity to AnimalsLD50: Not available. LC50: Not available. Chronic Effects on Humans Not available. Other Toxic Effects onSlightly hazardous in case of skin contact (irritant), of ingestion, of inhalation. Humans Special Remarks onNot available. Toxicity to Animals Special Remarks on May cause adverse reproductive effects and birth defects (teratogenic). Chronic Effects on Humans Excreted in maternal milk in human. Special Remarks on otherAcute Potential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. It may be absorbed through the skin. Eyes: Dust may cause eye irritation. Inhalation: Dust may cause respiratory tract irritation. Ingestion: May cause gastrointestinal tract irritation. May also affect behavior (toxic psychosis), metabolism (anorexia), and urinary system. This material is readily absorbed from the gastrointestinal tract. Ingestion of a massive single dose is unlikely to cause adverse effects. Chronic Potential Health Effects: Prolonged or repeated exposure by inhalation, ingestion, or skin contact may cause allergic reaction (possible hypersensitization). Other symptoms of chronic overdose effects may include, acne or other skin problems, weight loss or weight gain, hip or shoulder pain, fullness in face, fluid retention associated with sodium retention, potassium loss,swelling of feet or lower legs, excess or abnormal thirst, menstrual irregularities, nausea, vomiting, irregular heartbeat, muscle cramps, weakness, osteoporosis, increased susceptibility to infection, psychosis, and eye problems. May also affect urinary system (polyuria), liver, adrenal gland. Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. The product itself and its products of degradation are not toxic. Toxicity of the Products of Biodegradation Special Remarks on theNot available. Products of Biodegradation Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Prednisolone acetate Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). IdentificationNot applicable. Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms TSCA 8(b) inventory: Prednisolone acetate Federal and State Regulations CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found to cause cancer which would require a warning under the statute: No products were found. Proposition 65 Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: No products were found. Other RegulationsEINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS No. 200-134-1). Canada: Listed on Canadian Domestic Substance List (DSL). China: Not listed on National Inventory. Japan: Not listed on National Inventory (ENCS). Korea: Listed on National Inventory (KECI). Philippines: Mot listed on National Inventory (PICCS). Australia: Listed on AICS. WHMIS (Canada) CLASS D-2A: Material causing other toxic effects (VERY TOXIC). Other Classifications DSCL (EEC) R62- Possible risk of impaired fertility.S36- Wear suitable protective clothing. R63- Possible risk of harm to the unborn child. Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) Prednisolone acetate ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Wear appropriate respirator when ventilation is inadequate. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | TU4152500 |
| HS Code | 2937210000 |
|
~% 
Prednisolone Acetate CAS#:52-21-1 |
| Literature: Hogg et al. Journal of the American Chemical Society, 1955 , vol. 77, p. 4436 |
|
~% 
Prednisolone Acetate CAS#:52-21-1 |
| Literature: The Upjohn Company Patent: US5426198 A1, 1995 ; |
|
~% 
Prednisolone Acetate CAS#:52-21-1 |
| Literature: Herzog et al. Journal of the American Chemical Society, 1955 , vol. 77, p. 4781,4783 |
|
~% 
Prednisolone Acetate CAS#:52-21-1 |
| Literature: Florey; Restivo Journal of Organic Chemistry, 1957 , vol. 22, p. 406,408 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2937210000 |
|---|
|
Development and validation of a new stability indicating reversed phase liquid chromatographic method for the determination of prednisolone acetate and impurities in an ophthalmic suspension.
J. Pharm. Biomed. Anal. 102 , 261-6, (2015) A new stability indicating reversed phase high performance liquid chromatography (RP-HPLC) method was developed and validated under current International Conference of Harmonisation (ICH) guidance for... |
|
|
Convenient QSAR model for predicting the complexation of structurally diverse compounds with β-cyclodextrins
Bioorg. Med. Chem. 17 , 896-904, (2009) This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoret... |
|
|
Investigation of Overrun-Processed Porous Hyaluronic Acid Carriers in Corneal Endothelial Tissue Engineering.
PLoS ONE 10 , e0136067, (2015) Hyaluronic acid (HA) is a linear polysaccharide naturally found in the eye and therefore is one of the most promising biomaterials for corneal endothelial regenerative medicine. This study reports, fo... |
| Pregna-1,4-diene-3,20-dione, 21- (acetyloxy)-11,17-dihydroxy-, (11β)- |
| (11b)-21-(Acetyloxy)-11,17-dihydroxypregna-1,4-diene-3,20-dione |
| acétate de 2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-diméthyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodécahydro-3H-cyclopenta[a]phénanthrén-17-yl]-2-oxoéthyle |
| Scherisolon |
| EINECS 200-134-1 |
| Prednisolone acetate |
| 11b,17,21-Trihydroxypregna-1,4-diene-3,20-dione 21-acetate |
| Ak-Tate |
| Pregna-1,4-diene-3,20-dione, 11β,17,21-trihydroxy-, 21-acetate (8CI) |
| Prednisolone-21-acetate |
| MFCD00037710 |
| Deltacortenolo |
| (11β)-11,17-Dihydroxy-3,20-dioxopregna-1,4-dien-21-yl acetate |
| 2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl acetate |
| 2-[(8S,9S,10R,11S,13S,14S,17R)-11,17-Dihydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl]-2-oxoethylacetat |
| Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-11,17-dihydroxy-, (11β)- |
| Hydrocortidelt |
 CAS#:38680-83-0
CAS#:38680-83-0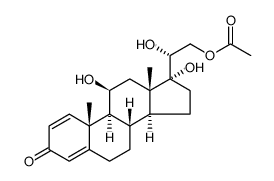 CAS#:96346-38-2
CAS#:96346-38-2![[(2S)-2-[(8S,9S,10S,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-hydroxy-ethyl] acetate structure](https://image.chemsrc.com/caspic/106/2871-71-8.png) CAS#:2871-71-8
CAS#:2871-71-8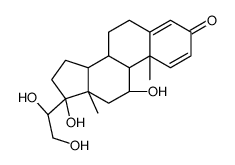 CAS#:15847-24-2
CAS#:15847-24-2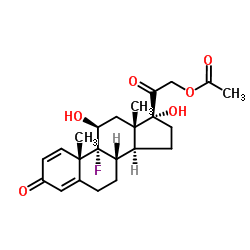 CAS#:338-98-7
CAS#:338-98-7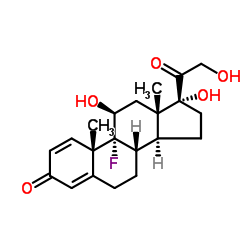 CAS#:338-95-4
CAS#:338-95-4 CAS#:79-20-9
CAS#:79-20-9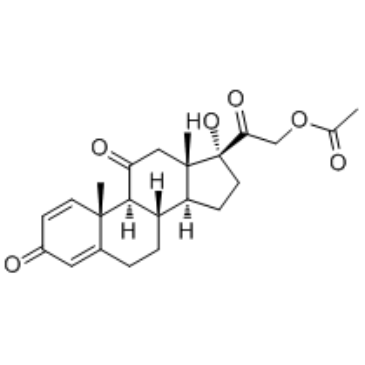 CAS#:125-10-0
CAS#:125-10-0![(8S,9S,10R,11S,13S,14S)-11-hydroxy-10,13-dimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-3,17-dione structure](https://image.chemsrc.com/caspic/329/898-84-0.png) CAS#:898-84-0
CAS#:898-84-0