prednisolone
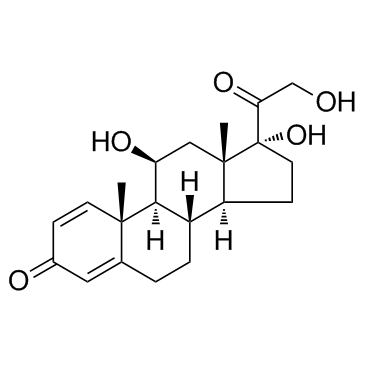
prednisolone structure
|
Common Name | prednisolone | ||
|---|---|---|---|---|
| CAS Number | 50-24-8 | Molecular Weight | 360.444 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 570.6±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H28O5 | Melting Point | 240 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 313.0±26.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of prednisolonePrednisolone is a glucocorticoid with the general properties of the corticosteroids.Target: Glucocorticoid ReceptorPrednisolone is a glucocorticoid with the general properties of the corticosteroids. It is the drug of choice for all conditions in which routine systemic corticosteroid therapy is indicated, except adrenal deficiency states. Prednisolone, 5 or 50 mg/kg, was administered intravenously to adrenalectomized rats. Total plasma, free plasma, CBG-free plasma, and liver prednisolone concentrations were measured simultaneously with free hepatic cytosolic glucocorticoid receptor concentrations and tyrosine aminotransferase (TAT) activity of the liver as a function of time. prednisolone pharmacokinetics were dose-dependent, parameters describing receptor kinetics and TAT activity were constant at each prednisolone dose. The major determinants of receptor-mediated glucocorticoid activity are confirmed to be the availability of the receptor, drug-receptor dissociation rate, and corticosteroid persistence in the biophase [1, 2]. |
| Name | prednisolone |
|---|---|
| Synonym | More Synonyms |
| Description | Prednisolone is a glucocorticoid with the general properties of the corticosteroids.Target: Glucocorticoid ReceptorPrednisolone is a glucocorticoid with the general properties of the corticosteroids. It is the drug of choice for all conditions in which routine systemic corticosteroid therapy is indicated, except adrenal deficiency states. Prednisolone, 5 or 50 mg/kg, was administered intravenously to adrenalectomized rats. Total plasma, free plasma, CBG-free plasma, and liver prednisolone concentrations were measured simultaneously with free hepatic cytosolic glucocorticoid receptor concentrations and tyrosine aminotransferase (TAT) activity of the liver as a function of time. prednisolone pharmacokinetics were dose-dependent, parameters describing receptor kinetics and TAT activity were constant at each prednisolone dose. The major determinants of receptor-mediated glucocorticoid activity are confirmed to be the availability of the receptor, drug-receptor dissociation rate, and corticosteroid persistence in the biophase [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 570.6±50.0 °C at 760 mmHg |
| Melting Point | 240 °C (dec.)(lit.) |
| Molecular Formula | C21H28O5 |
| Molecular Weight | 360.444 |
| Flash Point | 313.0±26.6 °C |
| Exact Mass | 360.193665 |
| PSA | 94.83000 |
| LogP | 1.50 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.612 |
| InChIKey | OIGNJSKKLXVSLS-VWUMJDOOSA-N |
| SMILES | CC12C=CC(=O)C=C1CCC1C2C(O)CC2(C)C1CCC2(O)C(=O)CO |
| Storage condition | -20°C Freezer |
|
Section 1. Chemical Product and Company Identification Prednisolone Common Name/ Trade Name Prednisolone Section 3. Hazards Identification Potential Acute Health Effects Slightly hazardous in case of skin contact (irritant, permeator), of eye contact (irritant), of ingestion, of inhalation.
Potential Chronic HealthSlightly hazardous in case of skin contact (sensitizer). EffectsCARCINOGENIC EFFECTS: Not available. MUTAGENIC EFFECTS: Mutagenic for mammalian somatic cells. Mutagenic for bacteria and/or yeast. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Classified Reproductive system/toxin/female, Reproductive system/toxin/male [POSSIBLE]. Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. WARM water MUST be used. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. Ingestion Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionThese products are carbon oxides (CO, CO2). Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various SubstancesNon-flammable in presence of shocks. Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. of Various SubstancesRisks of explosion of the product in presence of static discharge: Not available. Fire Fighting MediaSMALL FIRE: Use DRY chemical powder. and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Special Remarks onNot available. Fire Hazards Special Remarks on Explosion Not available. Hazards Prednisolone Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not ingest. Do not breathe dust. Wear suitable protective clothing. If ingested, seek medical advice immediately and show the container or the label. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Solid crystalline powder.)OdorOdorless. Not available. Taste Molecular Weight360.45 g/mole White. Off-white. Color pH (1% soln/water)Not available. Boiling PointNot available. Melting Point235°C (455°F) Critical TemperatureNot available. Specific GravityNot available. Not applicable. Vapor Pressure Vapor DensityNot available. Not available. Volatility Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Ionicity (in Water)Not available. Dispersion PropertiesSee solubility in water, methanol, acetone. SolubilitySoluble in acetone. Partially soluble in methanol. Very slightly soluble in cold water. Prednisolone Section 10. Stability and Reactivity Data The product is stable. Stability Instability TemperatureNot available. Excess heat, moisture Conditions of Instability Not available. Incompatibility with various substances CorrosivityNon-corrosive in presence of glass. Special Remarks onAvoid exposure to moisture Reactivity Special Remarks onNot available. Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryAbsorbed through skin. Inhalation. Ingestion. Toxicity to AnimalsAcute oral toxicity (LD50): 1680 mg/kg [Mouse]. Chronic Effects on HumansMUTAGENIC EFFECTS: Mutagenic for mammalian somatic cells. Mutagenic for bacteria and/or yeast. DEVELOPMENTAL TOXICITY: Classified Reproductive system/toxin/female, Reproductive system/toxin/male, Development toxin [POSSIBLE]. Other Toxic Effects onSlightly hazardous in case of skin contact (irritant, permeator), of ingestion, of inhalation. Humans Special Remarks on Not available. Toxicity to Animals Special Remarks onMay cause adverse reproductive effects (maternal effects on fertility -post-implantation, and fetotoxicity), and birth Chronic Effects on Humansdefects. May affect genetic material (mutagenic). Excreted in maternal milk in human. Special Remarks on otherAcute Potential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. It may be absorbed through the skin. Eyes: Dust may cause eye irritation. Inhalation: Dust may cause respiratory tract irritation. Ingestion: May cause gastrointestinal tract irritation. May also affect behavior (toxic psychosis), metabolism (anorexia), and urinary system. This material is readily absorbed from the gastrointestinal tract. Ingestion of a massive single dose is unlikely to cause adverse effects. Chronic Potential Health Effects: Prolonged or repeated exposure by inhalation, ingestion, or skin contact may cause allergic reaction (possible hypersensitization). Other symptoms of chronic overdose effects may include, acne or other skin problems, weight loss or weight gain, hip or shoulder pain, fullness in face, fluid retention associated with sodium retention, potassium loss,swelling of feet or lower legs, excess or abnormal thirst, menstrual irregularities, nausea, vomiting, irregular heartbeat, muscle cramps, weakness, osteoporosis, increased susceptibility to infection, psychosis, and eye problems. May also affect urinary system (polyuria), liver, adrenal gland. Prednisolone Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. The products of degradation are less toxic than the product itself. Toxicity of the Products of Biodegradation Special Remarks on theNot available. Products of Biodegradation Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). Not applicable. Identification Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms New Jersey: Prednisolone Federal and State TSCA 8(b) inventory: Prednisolone Regulations CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found Proposition 65to cause cancer which would require a warning under the statute: No products were found. Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: No products were found. Other RegulationsEINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS No. 200-021-7). Canada: Listed on Canadian Domestic Substance List (DSL). China: Listed on National Inventory. Japan: Not listed on National Inventory (ENCS). Korea: Listed on National Inventory (KECI). Philippines: Listed on National Inventory (PICCS). Australia: Listed on AICS. CLASS D-2A: Material causing other toxic effects (VERY TOXIC). Other ClassificationsWHMIS (Canada) Class D-2B: Material causing other toxic effects (TOXIC). DSCL (EEC)R62- Possible risk of impaired fertility.S36- Wear suitable protective clothing. R63- Possible risk of harm to the unborn child. Prednisolone Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Safety glasses. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P201-P280-P308 + P313 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TU4152000 |
| HS Code | 2937229000 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2937229000 |
|---|
|
Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing.
Eur. J. Pharm. Sci. 68 , 11-7, (2015) Rapid and reliable tailoring of the dose of controlled release tablets to suit an individual patient is a major challenge for personalized medicine. The aim of this work was to investigate the feasibi... |
|
|
Oral lactoferrin protects against experimental candidiasis in mice.
J. Appl. Microbiol. 118(1) , 212-21, (2014) To determine the role of human lactoferrin (hLF) in protecting the oral cavities of mice against Candida albicans infection in lactoferrin knockout (LFKO(-/-)) mice was compared to wild-type (WT) mice... |
|
|
Validation of cyclooxygenase-2 as a direct anti-inflammatory target of 4-O-methylhonokiol in zymosan-induced animal models.
Arch. Pharm. Res. 38 , 813-25, (2015) 4-O-methylhonokiol (MH) is known to inhibit inflammation by partially understood mechanisms. Here, the anti-inflammatory mechanisms of MH were examined using enzymatic, cellular, and animal assays. In... |
| Pregna-1,4-diene-3,20-dione, 11β,17,21-trihydroxy- |
| D1-Cortisol |
| 11b,17a,21-Trihydroxypregna-1,4-diene-3,20-dione |
| δ1-Dehydrocortisol |
| D1-Dehydrohydrocortisone |
| MFCD00003649 |
| (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one |
| 1,4-Pregnadiene-3,20-dione-11b,17a,21-triol |
| Deltasolone |
| δ-Stab |
| deltaf |
| 1,4-Pregnadiene-11b,17a,21-triol-3,20-dione |
| D1-Hydrocortisone |
| δ-Cortef |
| ulacort |
| Pregna-1,4-diene-3,20-dione, 11,17,21-trihydroxy-, (11β)- |
| eazolind |
| Precortalon |
| prednis |
| D1-Dehydrocortisol |
| solone |
| Flamasone |
| Klismacort |
| (11β)-11,17,21-Trihydroxypregna-1,4-diene-3,20-dione |
| Supercortisol |
| 3,20-Dioxo-11b,17a,21-trihydroxy-1,4-pregnadiene |
| d-Cortef |
| Deltisolone |
| d-Stab |
| NISOLONE |
| STEROLONE |
| d-Ef-Cortelan |
| prednisolone |
| δ1-Dehydrohydrocortisone |
| steran |
| dicortol |
| EINECS 200-021-7 |
| δ1-Hydrocortisone |
| hydeltra |
| predonin |
| (11b)-11,17,21-Trihydroxypregna-1,4-diene-3,20-dione |
| δ1-Cortisol |
| 11b,17,21-Trihydroxypregna-1,4-diene-3,20-dione |
 CAS#:98523-85-4
CAS#:98523-85-4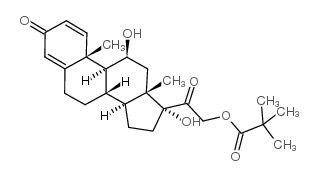 CAS#:1107-99-9
CAS#:1107-99-9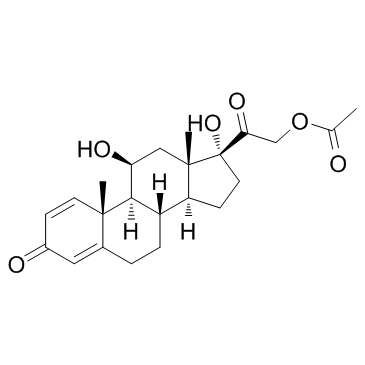 CAS#:52-21-1
CAS#:52-21-1 CAS#:67-56-1
CAS#:67-56-1![2-((8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl 3-(2-aminophenyl)propanoate Structure](https://image.chemsrc.com/caspic/439/1154703-42-0.png) CAS#:1154703-42-0
CAS#:1154703-42-0![2-((8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl 2-(2-nitrophenyl)acetate Structure](https://image.chemsrc.com/caspic/314/1426535-35-4.png) CAS#:1426535-35-4
CAS#:1426535-35-4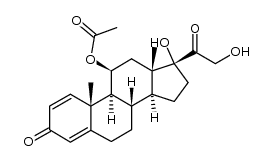 CAS#:104687-90-3
CAS#:104687-90-3 CAS#:50-23-7
CAS#:50-23-7 CAS#:2920-86-7
CAS#:2920-86-7![methyl 11-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-3-oxo-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-16-carboxylate structure](https://image.chemsrc.com/caspic/474/111802-43-8.png) CAS#:111802-43-8
CAS#:111802-43-8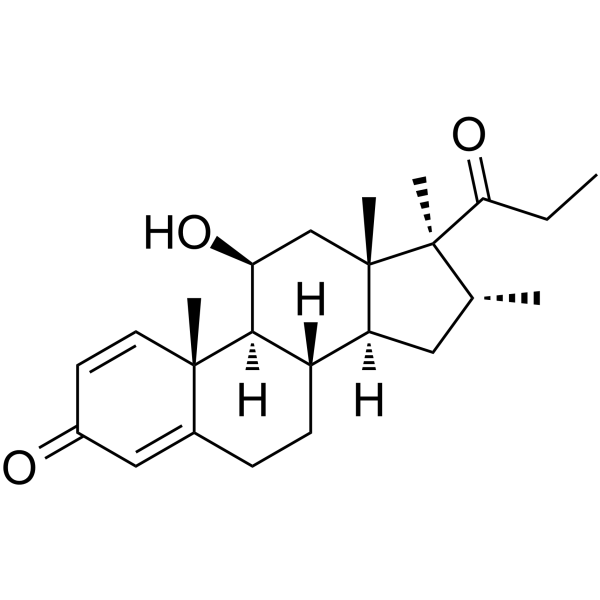 CAS#:49697-38-3
CAS#:49697-38-3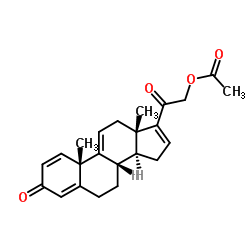 CAS#:37413-91-5
CAS#:37413-91-5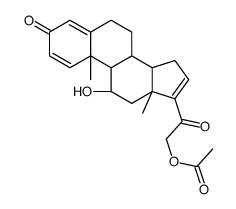 CAS#:3044-42-6
CAS#:3044-42-6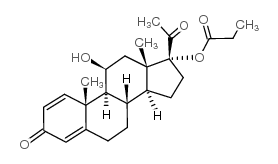 CAS#:20423-99-8
CAS#:20423-99-8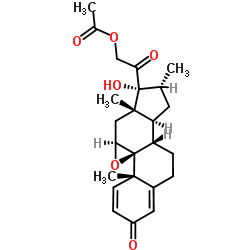 CAS#:2884-51-7
CAS#:2884-51-7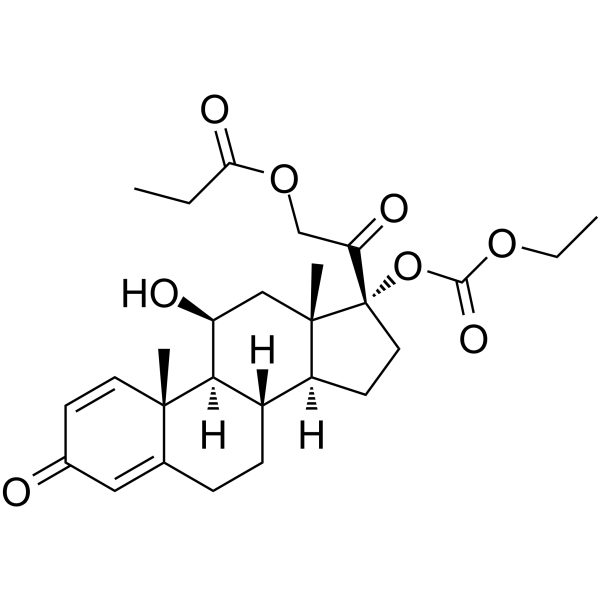 CAS#:73771-04-7
CAS#:73771-04-7![ethyl [(8S,9S,10R,11S,13S,14S,17R)-11-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl] carbonate structure](https://image.chemsrc.com/caspic/095/104286-02-4.png) CAS#:104286-02-4
CAS#:104286-02-4
