CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
TU3832200
-
CHEMICAL NAME :
-
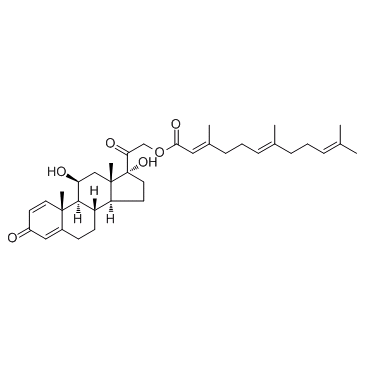
Pregna-1,4-diene-3,20-dione, 11,17-dihydroxy-21-((3,7,11-trimethyl-1-oxo-2,6,10- dodecatrienyl)-oxy)-, (11beta,21(2E,6E))-
-
CAS REGISTRY NUMBER :
-
118244-44-3
-
LAST UPDATED :
-
199306
-
DATA ITEMS CITED :
-
7
-
MOLECULAR FORMULA :
-
C36-H50-O6
-
MOLECULAR WEIGHT :
-
578.86
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1456 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Endocrine - changes in spleen weight Blood - changes in leukocyte (WBC) count Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 17(Suppl 3),49,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
728 mg/kg/52W-I
-
TOXIC EFFECTS :
-
Liver - hepatitis (hepatocellular necrosis), zonal Endocrine - changes in spleen weight Biochemical - Metabolism (Intermediary) - other proteins
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 17(Suppl 3),91,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2730 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Blood - changes in leukocyte (WBC) count Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases Related to Chronic Data - death
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 17(Suppl 3),1,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
291 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Endocrine - changes in adrenal weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Biochemical - Metabolism (Intermediary) - lipids including transport
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 17(Suppl 3),123,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
291 mg/kg/52W-I
-
TOXIC EFFECTS :
-
Endocrine - changes in adrenal weight Blood - changes in leukocyte (WBC) count Biochemical - Metabolism (Intermediary) - other proteins
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 17(Suppl 3),161,1992 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
>11 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - hepatobiliary system
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 17(Suppl 3),217,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
3250 ug/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 17(Suppl 3),241,1992
|