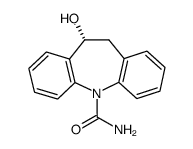
Licarbazepine
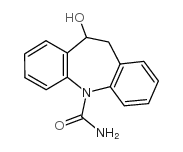
Licarbazepine structure
|
Common Name | Licarbazepine | ||
|---|---|---|---|---|
| CAS Number | 29331-92-8 | Molecular Weight | 254.28400 | |
| Density | 1.336g/cm3 | Boiling Point | 431.3ºC at 760mmHg | |
| Molecular Formula | C15H14N2O2 | Melting Point | 186-189ºC | |
| MSDS | Chinese | Flash Point | 214.6ºC | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of LicarbazepineLicarbazepine (BIA 2-005; GP 47779) is a voltage-gated sodium channel blocker with anticonvulsant and mood-stabilizing effects[1]. |
| Name | Licarbazepine |
|---|---|
| Synonym | More Synonyms |
| Description | Licarbazepine (BIA 2-005; GP 47779) is a voltage-gated sodium channel blocker with anticonvulsant and mood-stabilizing effects[1]. |
|---|---|
| Related Catalog | |
| Target |
Sodium Channel[1] |
| In Vivo | Eslicarbazepine acetate (ESL) is an oral pro-drug that is rapidly and extensively metabolized by the liver via a hydrolytic first-pass metabolism into S-Licarbazepine, the biologically active drug. The plasma level of the prodrug remains below quantification[1]. ESL is a potent antiepileptic agent with a spectrum of action essentially limited to partial-onset and generalized tonic-clonic seizures. Its main mechanism of action is by blocking the voltage-gated sodium channel. ESL works by blocking the voltage-gated sodium channel, which play an essential role in the generation and propagation of the epileptic discharge. ESL is well absorbed after oral administration with a bio-availability about 16% higher than that observed after an equivalent dose of Oxcarbazepine (OXC)[1]. |
| References |
| Density | 1.336g/cm3 |
|---|---|
| Boiling Point | 431.3ºC at 760mmHg |
| Melting Point | 186-189ºC |
| Molecular Formula | C15H14N2O2 |
| Molecular Weight | 254.28400 |
| Flash Point | 214.6ºC |
| Exact Mass | 254.10600 |
| PSA | 66.56000 |
| LogP | 3.25820 |
| Vapour Pressure | 3.33E-08mmHg at 25°C |
| Index of Refraction | 1.677 |
| InChIKey | BMPDWHIDQYTSHX-UHFFFAOYSA-N |
| SMILES | NC(=O)N1c2ccccc2CC(O)c2ccccc21 |
| Storage condition | Store at RT |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H332-H319 |
| Precautionary Statements | P210-P305 + P351 + P338 |
| Hazard Codes | N,Xn,F |
| Risk Phrases | 51/53-36-20/21/22-11 |
| Safety Phrases | 61-36/37-26-16 |
| RIDADR | UN 3077 9 / PGIII |
| HS Code | 2933990090 |
|
~90% 
Licarbazepine CAS#:29331-92-8 |
| Literature: KANDULA, Mahesh Patent: WO2013/167985 A1, 2013 ; Location in patent: Paragraph 00102; 00103 ; |
|
~91% 
Licarbazepine CAS#:29331-92-8 |
| Literature: Portela and Ca., S.A. Patent: EP1477480 A1, 2004 ; Location in patent: Page 6, 7 ; |
|
~91% 
Licarbazepine CAS#:29331-92-8 |
| Literature: RANBAXY LABORATORIES LIMITED; HIRPARA, Ketan; KHANDURI, Chandra, Has; SHARMA, Mukesh, Kumar Patent: WO2013/8194 A2, 2013 ; Location in patent: Page/Page column 12; 13 ; |
|
~68% 
Licarbazepine CAS#:29331-92-8 |
| Literature: Panunzio, Mauro; Campana, Eileen; Breviglieri, Gabriele Patent: US2008/221320 A1, 2008 ; Location in patent: Page/Page column 2 ; |
|
~% 
Licarbazepine CAS#:29331-92-8 |
| Literature: Tian, Maoqun; Abdelrahman, Aliaa; Weinhausen, Stephanie; Hinz, Sonja; Weyer, Stefanie; Dosa, Stefan; El-Tayeb, Ali; Mueller, Christa E. Bioorganic and Medicinal Chemistry, 2014 , vol. 22, # 3 p. 1077 - 1088 |
|
~% 
Licarbazepine CAS#:29331-92-8 |
| Literature: Kittelman, Matthias; Lattmann, Rene; Ghisalba, Preste Bioscience, Biotechnology, and Biochemistry, 1993 , vol. 57, # 9 p. 1589 - 1590 |
| Precursor 6 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
First MEPS/HPLC assay for the simultaneous determination of venlafaxine and O-desmethylvenlafaxine in human plasma.
Bioanalysis 6(22) , 3025-38, (2014) A new high-performance liquid chromatography-fluorescence detection assay based on microextraction by packed sorbent as sample preparation approach is described to quantify venlafaxine (VEN) and its m... |
|
|
Evaluation of the matrix effect of different sample matrices for 33 pharmaceuticals by post-column infusion.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 1000 , 84-94, (2015) Matrix effects that occur during quantitative measurement by liquid chromatography mass spectrometry specifically when using electrospray ionization are a widely recognized phenomenon. Sample matrix c... |
| 10,11-Dihydro-10-hydroxy-5H-dibenz[b,f]azepine-5-carboxamide,Licarbazepine |
| Licarbazepine,10,11-Dihydro-10-hydroxy-5H-dibenz(Z)[b,f]azepin-5-carboxamide |
| 10,11-Dihydro-10-hydroxy Carbamazepine |
| 10,11-Dihydro-10-hydroxycarbamazepine |
| 5H-Dibenz[b,f]azepine-5-carboxamide,10,11-dihydro-10-hydroxy- |


![5-Carbamoyl-5H-dibenz[b,f]azepinEN5-Cyano-10-hydroxy-10,11-dihydro-5H-dibenz[b,f]azepine structure](https://image.chemsrc.com/caspic/393/356760-08-2.png)
 CAS#:104746-03-4
CAS#:104746-03-4 CAS#:104746-04-5
CAS#:104746-04-5