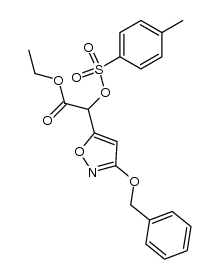
Ibotenic acid
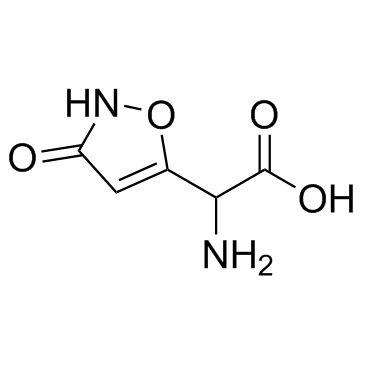
Ibotenic acid structure
|
Common Name | Ibotenic acid | ||
|---|---|---|---|---|
| CAS Number | 2552-55-8 | Molecular Weight | 158.112 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 458.8±45.0 °C at 760 mmHg | |
| Molecular Formula | C5H6N2O4 | Melting Point | 141-147 °C | |
| MSDS | Chinese USA | Flash Point | 231.3±28.7 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Ibotenic acidIbotenic acid has agonist activity at both the N-methyl-D-aspartate (NMDA) and trans-ACPD or metabolotropic quisqualate (Qm) receptor sites. |
| Name | Ibotenic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Ibotenic acid has agonist activity at both the N-methyl-D-aspartate (NMDA) and trans-ACPD or metabolotropic quisqualate (Qm) receptor sites. |
|---|---|
| Related Catalog | |
| Target |
NMDA receptor[1] |
| In Vitro | Ibotenic acid (Ibo) is capable of acting at both NMDA and trans-ACPD receptors in the CNS, although only activation of NMDA receptors is involved in Ibo neurotoxicity. Ibotenic acid is toxic to cortical neurons exposes for 5 min with an EC50=77.3±8 μM (n=5) as measured by release of lactate dehydrogenase to the culture media[1]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 458.8±45.0 °C at 760 mmHg |
| Melting Point | 141-147 °C |
| Molecular Formula | C5H6N2O4 |
| Molecular Weight | 158.112 |
| Flash Point | 231.3±28.7 °C |
| Exact Mass | 158.032761 |
| PSA | 109.58000 |
| LogP | -1.07 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.610 |
| InChIKey | IRJCBFDCFXCWGO-UHFFFAOYSA-N |
| SMILES | NC(C(=O)O)c1cc(=O)[nH]o1 |
| Storage condition | -20°C |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R23/24/25 |
| Safety Phrases | S45-S38-S36/37/39-S28A-S22 |
| RIDADR | 1544 |
| RTECS | NY2100000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
~% 
Ibotenic acid CAS#:2552-55-8 |
| Literature: Tetrahedron Letters, , p. 2081 - 2084 |
|
~% 
Ibotenic acid CAS#:2552-55-8 |
| Literature: Tetrahedron Letters, , p. 2081 - 2084 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Audition-independent vocal crystallization associated with intrinsic developmental gene expression dynamics.
J. Neurosci. 35(3) , 878-89, (2015) Complex learned behavior is influenced throughout development by both genetic and environmental factors. Birdsong, like human speech, is a complex vocal behavior acquired through sensorimotor learning... |
|
|
Assessment of sensorimotor gating following selective lesions of cholinergic pedunculopontine neurons.
Eur. J. Neurosci. 40(10) , 3526-37, (2014) Sensorimotor gating is the state-dependent transfer of sensory information into a motor system. When this occurs at an early stage of the processing stream it enables stimuli to be filtered out or par... |
|
|
Dopamine D2 Modulation of Sign and Goal Tracking in Rats.
Neuropsychopharmacology 40 , 2096-102, (2015) In Pavlovian conditioning, sign- and goal-tracking behaviors represent different approaches towards the conditioned stimulus. These behavioral patterns have been associated with predictive or incentiv... |
| Ibotenic acid |
| 5-Isoxazoleacetic acid, α-amino-2,3-dihydro-3-oxo- |
| a-Amino-3-hydroxy-5-isoxazoleacetic Acid |
| a-Amino-2,3-dihydro-3-oxo-5-isoxazoleacetic Acid |
| Acide amino(3-oxo-2,3-dihydro-1,2-oxazol-5-yl)acétique |
| Amino(3-oxo-2,3-dihydro-1,2-oxazol-5-yl)acetic acid |
| MFCD00069294 |
| Amino-(3-hydroxy-5-isoxazolyl)acetic acid |
| 2-amino-2-(3-oxo-1,2-oxazol-5-yl)acetic acid |