(Rac)-5-Hydroxymethyl Tolterodine
Modify Date: 2025-08-23 19:58:04
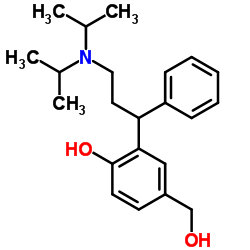
(Rac)-5-Hydroxymethyl Tolterodine structure
|
Common Name | (Rac)-5-Hydroxymethyl Tolterodine | ||
|---|---|---|---|---|
| CAS Number | 200801-70-3 | Molecular Weight | 341.49 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 490.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C22H31NO2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 233.2±27.4 °C | |
Use of (Rac)-5-Hydroxymethyl Tolterodine(Rac)-5-Hydroxymethyl Tolterodine ((Rac)-Desfesoterodine), an active metabolite of Tolterodine, is a mAChR antagonist (Ki values of 2.3 nM, 2 nM, 2.5 nM, 2.8 nM, and 2.9 nM for M1, M2, M3, M4, and M5 receptors, respectively). (Rac)-5-Hydroxymethyl Tolterodine can be used for overactive bladder research[1]. |
| Name | 2-[3-[di(propan-2-yl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenol |
|---|---|
| Synonym | More Synonyms |
| Description | (Rac)-5-Hydroxymethyl Tolterodine ((Rac)-Desfesoterodine), an active metabolite of Tolterodine, is a mAChR antagonist (Ki values of 2.3 nM, 2 nM, 2.5 nM, 2.8 nM, and 2.9 nM for M1, M2, M3, M4, and M5 receptors, respectively). (Rac)-5-Hydroxymethyl Tolterodine can be used for overactive bladder research[1]. |
|---|---|
| Related Catalog | |
| Target |
Ki: M1 (2.3 nM), M2 (2 nM), M3 (2.5 nM), M4 (2.8 nM), and M5 (2.9 nM)[1] |
| In Vitro | In vitro, (Rac)-5-Hydroxymethyl Tolterodine (PNU-200577) produces a competitive and concentration-dependent inhibition of carbachol-induced contraction of guinea-pig isolated urinary bladder strips (KB of 0.84 nM; pA2 of 9.14)[2]. |
| In Vivo | (Rac)-5-Hydroxymethyl Tolterodine (5-HMT; 0.88 μmol/kg; i.v.) treatment shows the binding activity of (Rac)-5-Hydroxymethyl Tolterodine to muscarinic receptors is significantly observed in all tissues, except cerebral cortex, with a longer duration in bladder[3]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 490.7±45.0 °C at 760 mmHg |
| Molecular Formula | C22H31NO2 |
| Molecular Weight | 341.49 |
| Flash Point | 233.2±27.4 °C |
| Exact Mass | 341.235474 |
| PSA | 43.70000 |
| LogP | 4.12 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.563 |
| InChIKey | DUXZAXCGJSBGDW-UHFFFAOYSA-N |
| SMILES | CC(C)N(CCC(c1ccccc1)c1cc(CO)ccc1O)C(C)C |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2922509090 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| Benzenemethanol, 3-[3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-hydroxy- |
| N,N-diisopropyl-3-(2-hydroxy-5-hydroxymethylphenyl)-3-phenylpropylamine |
| RS-N,N-diisopropyl-3-(2-hydroxy-5-(hydroxymethyl)phenyl)-3-phenylpropylamine |
| 2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethylphenol |
| 2-[3-(Diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)phenol |
| rac 5-Hydroxymethyl Tolterodine |
| feso deacyl raceme |
| (R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)phenol |
| 3-[3-[Bis(1-methylethyl)amino]-1-phenylpropyl]-4-hydroxybenzenemethanol |
| Fesoterodine fumarate Intermediate 1 |
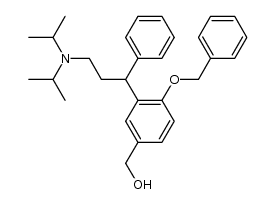 CAS#:250214-39-2
CAS#:250214-39-2 CAS#:14371-10-9
CAS#:14371-10-9 CAS#:623-05-2
CAS#:623-05-2 CAS#:156755-23-6
CAS#:156755-23-6 CAS#:250214-37-0
CAS#:250214-37-0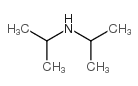 CAS#:108-18-9
CAS#:108-18-9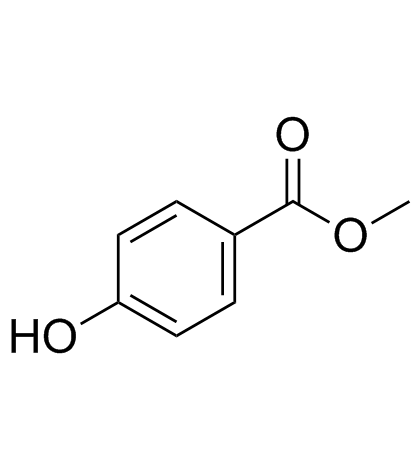 CAS#:99-76-3
CAS#:99-76-3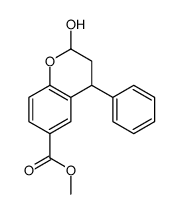 CAS#:380636-44-2
CAS#:380636-44-2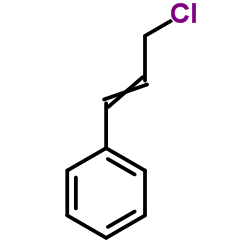 CAS#:2687-12-9
CAS#:2687-12-9 CAS#:207679-81-0
CAS#:207679-81-0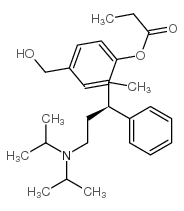 CAS#:286930-02-7
CAS#:286930-02-7