207679-81-0
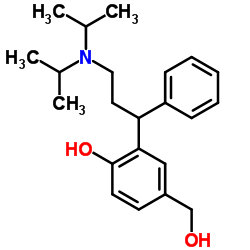
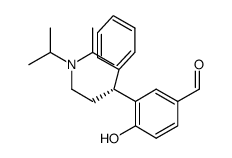
| Name | 5-hydroxymethyl Tolterodine |
|---|---|
| Synonyms |
(R)-2-(3-(Diisopropylamino)-1-phenylpropyl)-4-(hydroxymethyl)phenol
2-[(1R)-3-(Diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)phenol (R)-5-Hydroxymethyl tolterodine 5-hydroxymethyl tolterodine (PNU 200577) (R)-HYDROXYTOLTERODINE-D14 (R)-5-HYDROXYTOLTERODINE R-(+)-2(3-DIISOPROPYLAMINO-1-PHENYLPROPYL)-4-HYDROXYMETHYLPHENOL R-(+)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethylphenol R-5-Hydroxymethyl Tolterodine 3-[(1R)-3-[Bis(1-Methylethyl)Amino]-1-Phenylpropyl]-4-Hydroxy-Benzenemethanol Desfesoterodine 3-[(1R)-3-[BIS(1-METHYLETHYL)AMINO]-1-PHENYLPROPYL]-4-HYDROXYBENZENEMETHANOL Fesoterodine fumarate Intermediate 2 Fesoterodine Impurity 1 |
| Description | (R)-5-Hydroxymethyl Tolterodine(PNU-200577; Desfesoterodine) is a potent and selective muscarinic receptor antagonist with a Kb and a pA2 of 0.84 nM and 9.14, respectively. IC50 value: 0.84 nM (Kb)Target: mAChR(R)-5-Hydroxymethyl Tolterodine is a major pharmacologically active metabolite of tolterodine. In vitro, (R)-5-Hydroxymethyl Tolterodine prevented carbachol-induced contraction of guinea-pig isolated urinary bladder strips in a competitive and concentration-dependent manner. In vivo, (R)-5-Hydroxymethyl Tolterodine was significantly more potent at suppressing acetylcholine-induced urinary bladder contraction than electrically induced salivation in the anaesthetised cat (ID50=15 and 40 nmol/kg, respectively). In radioligand binding studies carried out in homogenates of guinea-pig tissues and Chinese hamster ovary cell lines expressing human muscarinic m1-m5 receptors, (R)-5-Hydroxymethyl Tolterodine was not selective for any muscarinic receptor subtype. Thus, (R)-5-Hydroxymethyl Tolterodine is similar to tolterodine in terms of antimuscarinic potency, functional selectivity for the urinary bladder in vivo and absence of selectivity for muscarinic receptor subtypes in vitro. The results of this study clearly indicate that (R)-5-Hydroxymethyl Tolterodine contributes to the therapeutic action of tolterodine, in view of its high antimuscarinic potency, similar serum concentration and lower degree of protein binding. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 490.7±45.0 °C at 760 mmHg |
| Melting Point | 68-72°C |
| Molecular Formula | C22H31NO2 |
| Molecular Weight | 341.487 |
| Flash Point | 233.2±27.4 °C |
| Exact Mass | 341.235474 |
| PSA | 43.70000 |
| LogP | 4.12 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.563 |
| Storage condition | -20?C Freezer |
| HS Code | 2922509090 |
|---|
| Precursor 7 | |
|---|---|
| DownStream 3 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |

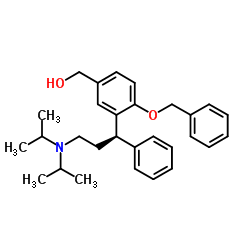
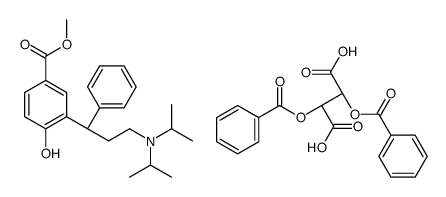
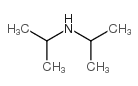
![(R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-(hydroxymethyl)phenol (R)-2-acetoxy(phenyl)acetate structure](https://image.chemsrc.com/caspic/384/1333234-72-2.png)
![3-[(1R)-3-(Diisopropylamino)-1-phenylpropyl]-4-hydroxybenzenemethanol (2E)-2-butenedioate structure](https://image.chemsrc.com/caspic/357/380636-50-0.png)