Bimatoprost

Bimatoprost structure
|
Common Name | Bimatoprost | ||
|---|---|---|---|---|
| CAS Number | 155206-00-1 | Molecular Weight | 415.566 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 629.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C25H37NO4 | Melting Point | 66-68°C | |
| MSDS | USA | Flash Point | 334.7±31.5 °C | |
Use of BimatoprostBimatoprost is a prostaglandin analog used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension.Target: Prostaglandin ReceptorBimatoprost is a prostaglandin analog/prodrug used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension. It reduces intraocular pressure (IOP) by increasing the outflow of aqueous fluid from the eyes. In December 2008, the indication to lengthen eyelashes was approved by the U.S. Food and Drug Administration (FDA); the cosmetic formulation of bimatoprost is sold as Latisse. In 2008-2011, at least three case series suggested that bimatoprost has the ability to reduce adipose (fat) tissue.Bimatoprost activates prostamide alpha F2 receptors found in the hair follicle to stimulate its growth rate. Research led by Professor Randall and the University of Bradford found that it may also offer a treatment for scalp hair regrowth in trials conducted on samples taken from men undergoing hair transplants. According to Allergan's package labeling, users of its Latisse cosmetic product didn't develop darker irises in clinical studies; however, "patients should be advised about the potential for increased brown iris pigmentation which is likely to be permanent." |
| Name | bimatoprost |
|---|---|
| Synonym | More Synonyms |
| Description | Bimatoprost is a prostaglandin analog used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension.Target: Prostaglandin ReceptorBimatoprost is a prostaglandin analog/prodrug used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension. It reduces intraocular pressure (IOP) by increasing the outflow of aqueous fluid from the eyes. In December 2008, the indication to lengthen eyelashes was approved by the U.S. Food and Drug Administration (FDA); the cosmetic formulation of bimatoprost is sold as Latisse. In 2008-2011, at least three case series suggested that bimatoprost has the ability to reduce adipose (fat) tissue.Bimatoprost activates prostamide alpha F2 receptors found in the hair follicle to stimulate its growth rate. Research led by Professor Randall and the University of Bradford found that it may also offer a treatment for scalp hair regrowth in trials conducted on samples taken from men undergoing hair transplants. According to Allergan's package labeling, users of its Latisse cosmetic product didn't develop darker irises in clinical studies; however, "patients should be advised about the potential for increased brown iris pigmentation which is likely to be permanent." |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 629.8±55.0 °C at 760 mmHg |
| Melting Point | 66-68°C |
| Molecular Formula | C25H37NO4 |
| Molecular Weight | 415.566 |
| Flash Point | 334.7±31.5 °C |
| Exact Mass | 415.272247 |
| PSA | 89.79000 |
| LogP | 1.98 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.591 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis.
Dermatol. Surg. 40(10) , 1118-24, (2014) The efficacy and safety of bimatoprost ophthalmic solution 0.03% for treating hypotrichosis were shown in a randomized controlled trial and in an open-label study. To date, no data on real-world exper... |
|
|
Conjunctiva-associated lymphoid tissue (CALT) reactions to antiglaucoma prostaglandins with or without BAK-preservative in rabbit acute toxicity study.
PLoS ONE 7 , e33913, (2012) Conjunctiva-associated lymphoid tissue (CALT) is closely associated with ocular surface immunity. This study investigated the effects of antiglaucoma prostaglandin analogs with or without benzalkonium... |
|
|
Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes.
Mol. Vis. 18 , 431-8, (2012) To determine the proinflammatory cytokine profile of aqueous humor from glaucomatous eyes.Aqueous humor samples were prospectively collected from 38 eyes (26 primary open angle glaucoma [POAG] and 12 ... |
| (5Z)-7-{(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]cyclopentyl}-N-ethyl-5-heptenamide |
| BiMatoprost HOUSE STANDARD |
| BiMapnost |
| (5Z)-7-{(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenylpent-1-en-1-yl]cyclopentyl}-N-ethylhept-5-enamide |
| Prostamide |
| 17-Phenyl-tri-norprostaglandin F2α-ethyl amide |
| MFCD03411999 |
| 5-Heptenamide, 7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-penten-1-yl]cyclopentyl]-N-ethyl-, (5Z)- |
| Lumigan |
| Bimatoprost |
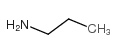 CAS#:107-10-8
CAS#:107-10-8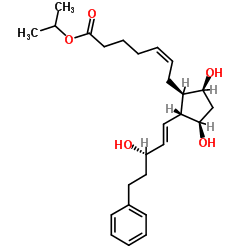 CAS#:130209-76-6
CAS#:130209-76-6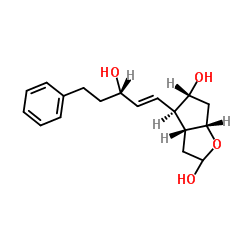 CAS#:856240-62-5
CAS#:856240-62-5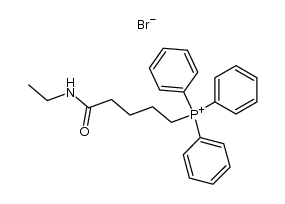 CAS#:1201226-16-5
CAS#:1201226-16-5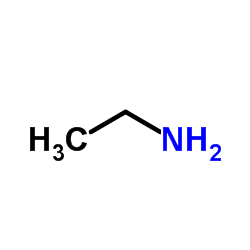 CAS#:75-04-7
CAS#:75-04-7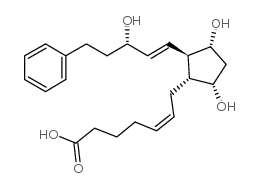 CAS#:38344-08-0
CAS#:38344-08-0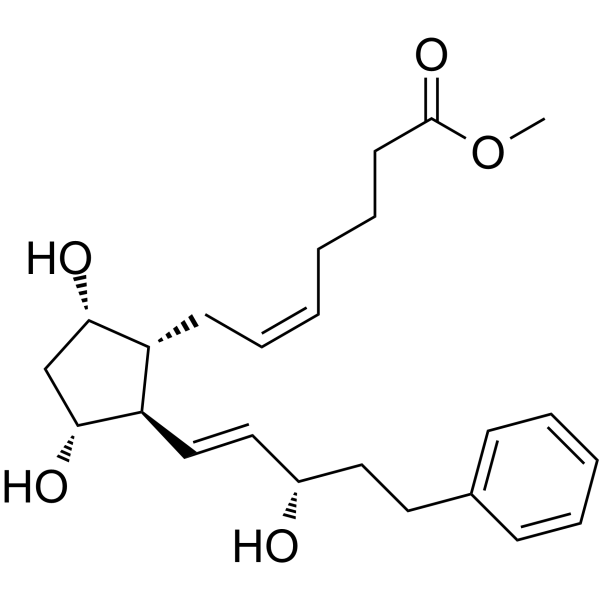 CAS#:38315-47-8
CAS#:38315-47-8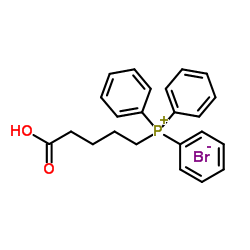 CAS#:17814-85-6
CAS#:17814-85-6