Pamoic acid
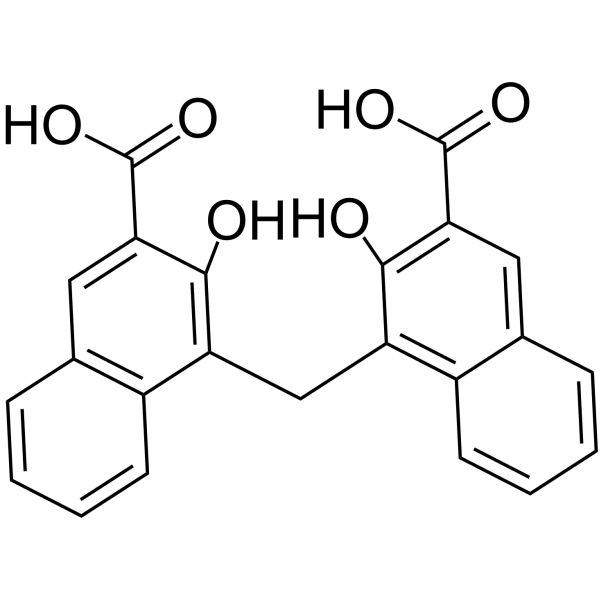
Pamoic acid structure
|
Common Name | Pamoic acid | ||
|---|---|---|---|---|
| CAS Number | 130-85-8 | Molecular Weight | 388.38 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 642.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C23H16O6 | Melting Point | ≥300 °C (dec.) | |
| MSDS | Chinese USA | Flash Point | 356.5±28.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Pamoic acidPamoic acid is a potent GPR35 agonist with an EC50 of 79 nM. Pamoic acid exhibits neuroprotective and anti-inflammatory properties[1][2]. |
| Name | pamoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Pamoic acid is a potent GPR35 agonist with an EC50 of 79 nM. Pamoic acid exhibits neuroprotective and anti-inflammatory properties[1][2]. |
|---|---|
| Related Catalog | |
| Target |
EC50: 79 nM (GPR35)[1] |
| In Vitro | GPR35 activation by Pamoic acid may increase the phosphorylation of ERK1/2, which in turn initiates an anti-inflammatory signal by suppressing NF-κB-dependent inflammatory genes[1]. |
| In Vivo | In a mouse model of stroke, that GPR35 activation by Pamoic acid (s.c.; 50-100 mg/kg) is neuroprotective. Pharmacological inhibition of GPR35 reveals that Pamoic acid reduces infarcts size in a GPR35 dependent manner. Pamoic acid treatment results in a preferential increment of noninflammatory Ly-6CLo monocytes/macrophages in the ischemic brain along with the reduced neutrophil counts[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 642.7±55.0 °C at 760 mmHg |
| Melting Point | ≥300 °C (dec.) |
| Molecular Formula | C23H16O6 |
| Molecular Weight | 388.38 |
| Flash Point | 356.5±28.0 °C |
| Exact Mass | 388.094696 |
| PSA | 115.06000 |
| LogP | 6.35 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.782 |
| InChIKey | WLJNZVDCPSBLRP-UHFFFAOYSA-N |
| SMILES | O=C(O)c1cc2ccccc2c(Cc2c(O)c(C(=O)O)cc3ccccc23)c1O |
| Water Solubility | PRACTICALLY INSOLUBLE |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | QL2180000 |
| HS Code | 2917399090 |
|
~95% 
Pamoic acid CAS#:130-85-8 |
| Literature: Journal of Organic Chemistry, , vol. 61, # 11 p. 3865 - 3869 |
|
~% 
Pamoic acid CAS#:130-85-8 |
| Literature: Chemische Berichte, , vol. 34, p. 4148 Chemische Berichte, , vol. 25, p. 3215 Chemische Berichte, , vol. 61, p. 1001 |
| HS Code | 2918290000 |
|---|---|
| Summary | HS: 2918290000 other carboxylic acids with phenol function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) VAT:17.0% MFN tariff:6.5% General tariff:30.0% |
|
Long-acting atypical antipsychotics: characterization of the local tissue response.
Pharm. Res. 31(8) , 2065-77, (2014) Long-acting injectables (LAIs) are increasingly recognized as an effective therapeutic approach for treating chronic conditions. Many LAIs are formulated to create a poorly soluble depot from which th... |
|
|
Identification of small-molecule antagonists that inhibit an activator: coactivator interaction.
Proc. Natl. Acad. Sci. U. S. A. 101 , 17622-17627, (2004) Phosphorylation of the cAMP response element binding protein (CREB) at Ser-133 in response to hormonal stimuli triggers cellular gene expression via the recruitment of the histone acetylase coactivato... |
|
|
Formation of stable nanocarriers by in situ ion pairing during block-copolymer-directed rapid precipitation.
Mol. Pharm. 10(1) , 319-28, (2013) We present an in situ hydrophobic salt forming technique for the encapsulation of weakly hydrophobic, ionizable active pharmaceutical ingredients (API) into stable nanocarriers (NCs) formed via a rapi... |
| Pamoic |
| 1,1'-Methylene-bis(2-hydroxy-3-naphthoic acid) |
| 2-Naphthalenecarboxylic acid, 4,4'-methylenebis[3-hydroxy- |
| 4,4-Methylenebis(3-Hydroxy-2-Naphthoic Acid) |
| EMBIONIC ACID |
| PAMOIC ACID FREE ACID |
| 2,2'-Dihydroxy-1,1'-dinaphthylmethane-3,3'-dicarboxylic Acid |
| 4,4'-Methylenebis(3-hydroxy-2-naphthoic acid) |
| Pamoic acid |
| PAMOIC ACID pure |
| Embonic acid |
| 4,4'-Methylenedi(3-hydroxy-2-naphthoic Acid) |
| EINECS 204-998-0 |
| MFCD00004079 |


 CAS#:6640-22-8
CAS#:6640-22-8 CAS#:10075-24-8
CAS#:10075-24-8![methyl 3-methoxy-4-[(2-methoxy-3-methoxycarbonylnaphthalen-1-yl)methyl]naphthalene-2-carboxylate structure](https://image.chemsrc.com/caspic/068/49609-90-7.png) CAS#:49609-90-7
CAS#:49609-90-7
