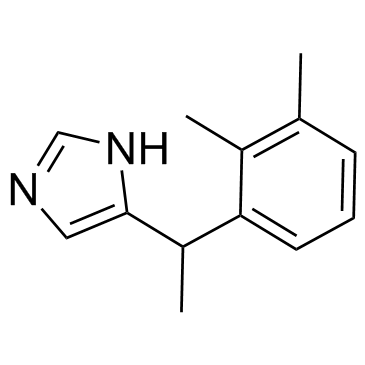
86347-15-1
| Name | medetomidine hydrochloride |
|---|---|
| Synonyms |
4-[(a-Methyl)-2,3-dimethylbenzyl]imidazole Monohydrochloride
4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole hydrochloride (1:1) 1H-Imidazole, 4-[1-(2,3-dimethylphenyl)ethyl]-, hydrochloride (1:1) (±)-4-(a,2,3-Trimethylbenzyl)imidazole Monohydrochloride 1H-Imidazole, 5-[1-(2,3-dimethylphenyl)ethyl]-, hydrochloride (1:1) medetomidine HCl UNII:BH210P244U 4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole Monohydrochloride Medetomidine (hydrochloride) |
| Description | Medetomidine Hydrochloride is an agonist of adrenergic alpha-2 receptor, which is used in veterinary medicine for its analgesic and sedative properties.Target: Adrenergic alpha-2 ReceptorMedetomidine, acting at alpha(2A) adrenoceptors, must be present during the encoding process to decrease discrete cue fear memory; however, its ability to suppress contextual memory is likely the result of blocking the consolidation process [1]. Medetomidine had no analgesic effects in alpha(2A)-adrenoceptor KO mice [2]. Medetomidine was effective in blocking these sympathomimetic actions of cocaine even in all 7 subjects who were homozygous for the Del322-325 polymorphism in the alpha2C AR, a loss-of-function mutation that is highly enriched in blacks [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 381.9ºC at 760 mmHg |
|---|---|
| Melting Point | 164-166°C |
| Molecular Formula | C13H17ClN2 |
| Molecular Weight | 236.740 |
| Flash Point | 191.3ºC |
| Exact Mass | 236.108032 |
| PSA | 28.68000 |
| LogP | 3.98030 |
| Storage condition | Refrigerator |
| HS Code | 2942000000 |
|---|
|
~89% 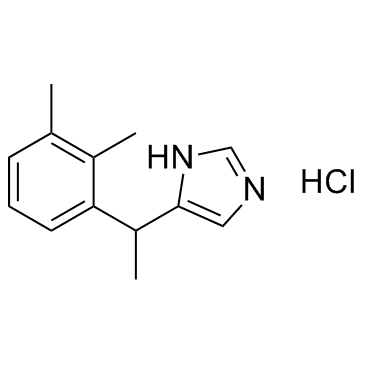
86347-15-1 |
| Literature: "Joint Stock Company Grindeks" Patent: EP1918282 A1, 2008 ; Location in patent: Page/Page column 6 ; |
|
~% 
86347-15-1 |
| Literature: WO2009/53709 A1, ; Page/Page column 13; 15-16 ; |
|
~% 
86347-15-1 |
| Literature: WO2013/14428 A1, ; |
|
~% 
86347-15-1 |
| Literature: WO2013/14428 A1, ; |
|
~% 
86347-15-1 |
| Literature: WO2013/14428 A1, ; |
| Precursor 3 | |
|---|---|
| DownStream 1 | |
| HS Code | 2942000000 |
|---|