4707-32-8
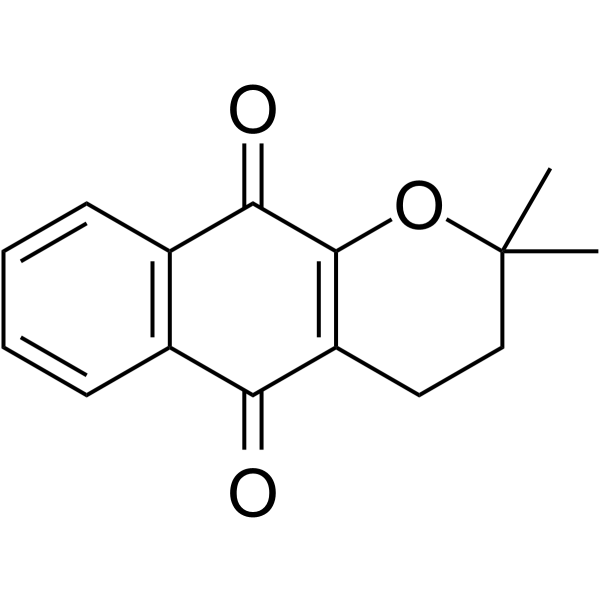
| Name | β-lapachone |
|---|---|
| Synonyms |
3,4-Dihydro-2,2-dimethyl-2H-naphtho(1,2-b)pyran-5,6-dione
Beta-Lapachone 3,4-Dihydro-2,2-dimethyl-2H-naphtho[2,3-b]pyran-5,10-dione β-lapachone 3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]-pyran-5,6-dione 2,2-Dimethyl-3,4-dihydro-2H-benzo[h]chromene-5,6-dione 2,2-dimethyl-3,4-dihydrobenzo[h]chromene-5,6-dione ARQ-501 CO-501 |
| Description | β-Lapachone is a naturally occurring O-naphthoquinone, acts as a topoisomerase I inhibitor, and induces apoptosis by inhibiting cell cycle progression. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I |
| In Vitro | β-Lapachone is a topoisomerase I inhibitor. β-Lapachone (25 μM) inhibits camptothecin-induced DNA cleavage[1]. β-Lapachone (10-40 μM) significantly reduces the colony-forming ability of CHO cells, and is cytotoxic in S phase. β-Lapachone at above 10 μM, causes a heavy DNA-strand breaks in CHO cells[2]. β-Lapachone (10 μM) suppresses JCPyV replication in IMR-32 cells. β-Lapachone (1.0 µM) potently affects JCPyV propagation in JCI cells. β-Lapachone (0.01-0.1 μM) inhibits VP1 production in JCI cells[4]. |
| In Vivo | β-Lapachone (0.066%) ameliorates cisplatin-induced renal injury and when in combination with cisplatin, the affect is more significant in mice. β-Lapachone increases Mre11-Rad50-Nbs1 (MRN) complex expression in mice[3]. |
| Kinase Assay | DNA topoisomerase I is incubated in the presence or absence of drugs (including β-Lapachone), in 20 μL of relaxation buffer (50 mM Tris (pH 7.5). 50 mM KCl, 10 mM MgCl2, 0.5 mM dithiothreitol, 0.5 mM EDTA, 30 μg/mL bovine serum albumin) for 30 min at 37°C. Reactions are stopped by adding 1% SDS and proteinase K (50 μg/mL). After an additional 1-h incubation at 37°C, the products are separated by electrophoresis in 1% agarose gel in TAE buffer (0.04 M tris acetate, 0.001 M EDTA). The gel is stained with ethidium bromide after electrophoresis. The photographic negative is scanned with an NIH image analysis system[1]. |
| Cell Assay | Cytotoxicity is measured by an MTT assay. IMR-32 and JCI cells are plated in 96-well microtiter plates at a concentration of 5.0 × 104 (topotecan) or 2.5 × 104 (β-lapachone) cells/well/100 µL medium 24 hr prior to addition of various concentrations of topotecan or β-lapachone. The cells are then incubated for 72 hr at 37°C in a CO2 incubator. Cell proliferation is assessed using a Cell Proliferation Kit I. Experiments are performed using four independent cultures[4]. |
| Animal Admin | Male Balb/c mice are provided a commercial pellet diet and water ad libitum. After 1 week of acclimation, the mice are randomly allocated to one of the following groups (5 per group): control, β-lapachone, cisplatin (18 mg/kg, ip), and β-lapachone + cisplatin (18 mg/kg, ip). The β-lapachone groups are fed a diet containing the drug (0.066) for 2 weeks prior to cisplatin injection. All mice are sacrificed under carbon dioxide anesthesia 3 days after cisplatin injection. The blood samples are subjected to serum BUN and CRE analyses. Half of the kidney is quickly removed for histopathological and immunohistochemical (IHC) studies. The other half is stored at −70°C until western blot assay[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 381.4±42.0 °C at 760 mmHg |
| Melting Point | >110ºC (dec.) |
| Molecular Formula | C15H14O3 |
| Molecular Weight | 242.270 |
| Flash Point | 169.7±27.9 °C |
| Exact Mass | 242.094299 |
| PSA | 43.37000 |
| LogP | 2.82 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.595 |
| Storage condition | -20?C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | QL6127400 |
| HS Code | 2914399090 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2914399090 |
|---|---|
| Summary | 2914399090. other aromatic ketones without other oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |

![Naphth[2,3-b]oxirene-2,7-dione, 1a,7a-dihydro-1a-(3-methyl-2-buten-1-yl) structure](https://image.chemsrc.com/caspic/083/137492-06-9.png)


![6-METHOXY-2,2-DIMETHYL-2H-BENZO[H]CHROMENE structure](https://image.chemsrc.com/caspic/308/573-13-7.png)

![2,2-Dimethyl-3,4-dihydro-2H-benzo[h]chromene structure](https://image.chemsrc.com/caspic/480/16274-33-2.png)
![5,6-dihydro-7,7-dimethyl-7H-pyrano[3',2':4]naphtha[1,2-b]pyrazine structure](https://image.chemsrc.com/caspic/040/833122-18-2.png)