| Structure | Name/CAS No. | Articles |
|---|---|---|
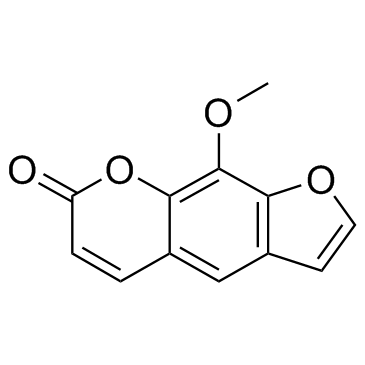 |
Xanthotoxin
CAS:298-81-7 |
|
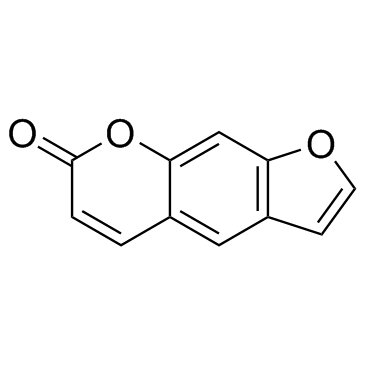 |
Psoralen
CAS:66-97-7 |
|
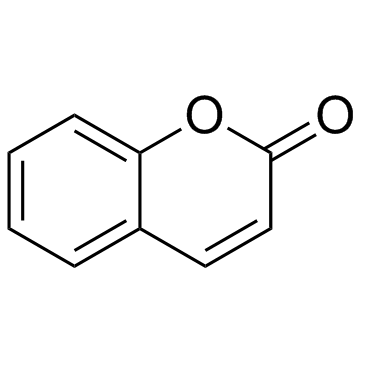 |
Coumarin
CAS:91-64-5 |
|
 |
7-Hydroxycoumarine
CAS:93-35-6 |
|
 |
Bergapten
CAS:484-20-8 |
|
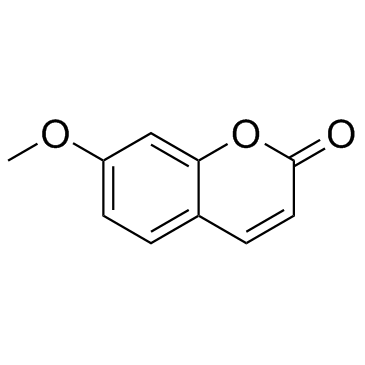 |
7-Methoxycoumarin
CAS:531-59-9 |
|
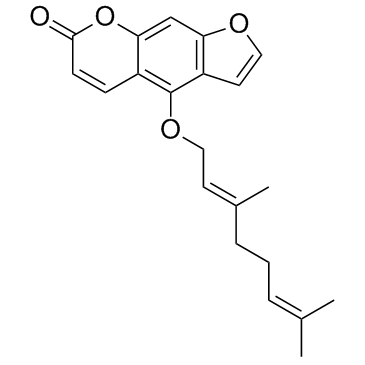 |
Bergamotine
CAS:7380-40-7 |
|
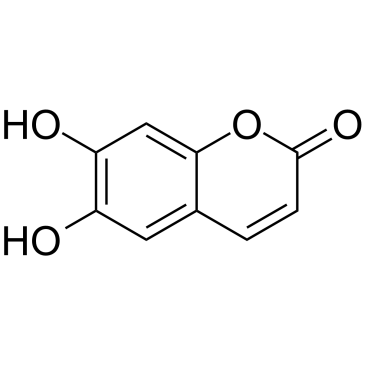 |
6,7-Dihydroxycoumarin
CAS:305-01-1 |