| Structure | Name/CAS No. | Articles |
|---|---|---|
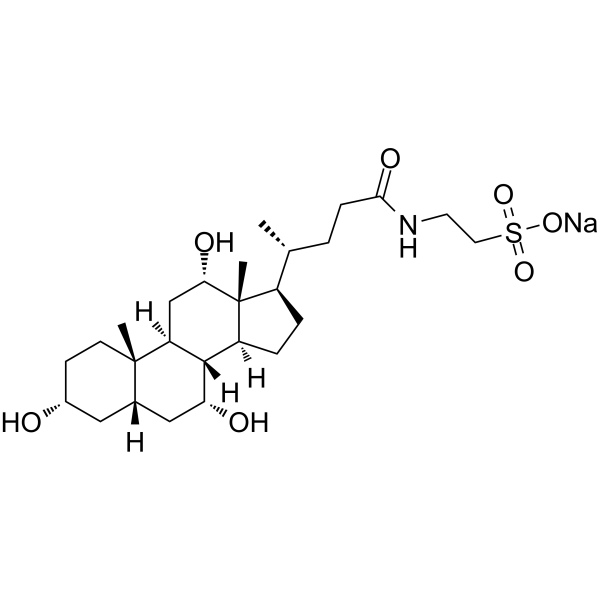 |
Sodium taurocholate
CAS:145-42-6 |
|
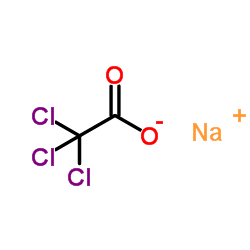 |
Sodium TCA
CAS:650-51-1 |
|
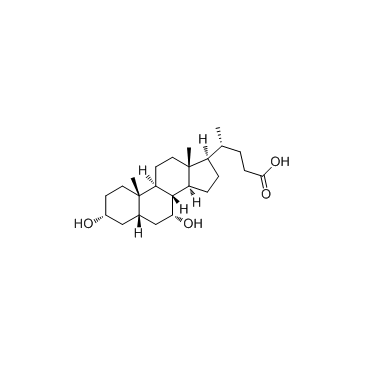 |
Chenodeoxycholic acid
CAS:474-25-9 |
|
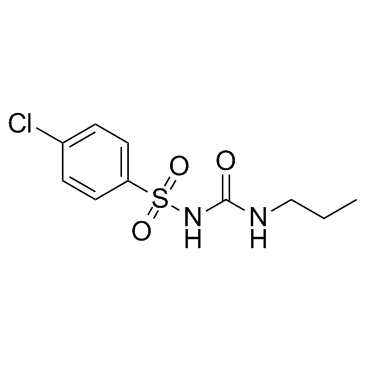 |
chlorpropamide
CAS:94-20-2 |
|
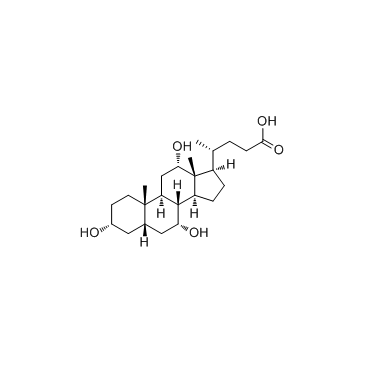 |
cholic acid
CAS:81-25-4 |
|
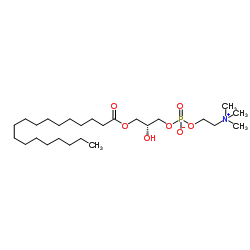 |
1-Stearoyl-sn-glycero-3-phosphocholine
CAS:19420-57-6 |