chlorpropamide
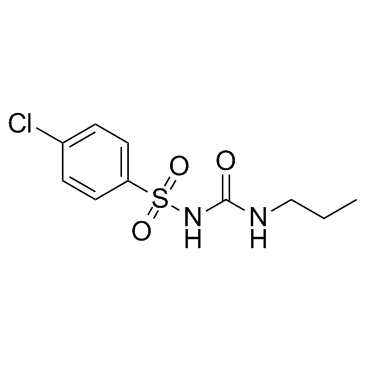
chlorpropamide structure
|
Common Name | chlorpropamide | ||
|---|---|---|---|---|
| CAS Number | 94-20-2 | Molecular Weight | 276.740 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 433.5±47.0 °C at 760 mmHg | |
| Molecular Formula | C10H13ClN2O3S | Melting Point | 128 °C | |
| MSDS | Chinese USA | Flash Point | 216.0±29.3 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of chlorpropamideChlorpropamide is an oral antihyperglycemic agent used for the treatment of non-insulin-dependent diabetes mellitus (NIDDM).Target:Chlorpropamide belongs to the sulfonylurea class of insulin secretagogues, which act by stimulating β cells of the pancreas to release insulin.Chlorpropamide is not recommended for the treatment of NIDDM as it increases blood pressure and the risk of retinopathy. Up to 80% of the single oral dose of chlorpropramide is metabolized, likely in the liver; 80-90% of the dose is excreted in urine as unchanged drug and metabolites. |
| Name | chlorpropamide |
|---|---|
| Synonym | More Synonyms |
| Description | Chlorpropamide is an oral antihyperglycemic agent used for the treatment of non-insulin-dependent diabetes mellitus (NIDDM).Target:Chlorpropamide belongs to the sulfonylurea class of insulin secretagogues, which act by stimulating β cells of the pancreas to release insulin.Chlorpropamide is not recommended for the treatment of NIDDM as it increases blood pressure and the risk of retinopathy. Up to 80% of the single oral dose of chlorpropramide is metabolized, likely in the liver; 80-90% of the dose is excreted in urine as unchanged drug and metabolites. |
|---|---|
| Related Catalog |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 433.5±47.0 °C at 760 mmHg |
| Melting Point | 128 °C |
| Molecular Formula | C10H13ClN2O3S |
| Molecular Weight | 276.740 |
| Flash Point | 216.0±29.3 °C |
| Exact Mass | 276.033539 |
| PSA | 83.65000 |
| LogP | 2.80 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.585 |
| Stability | Stable. Combustible. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H312-H332-H351 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | YS6650000 |
| HS Code | 2935009090 |
|
~92% 
chlorpropamide CAS#:94-20-2 |
| Literature: Tan, Davin; Strukil, Vjekoslav; Mottillo, Cristina; Friscic, Tomislav Chemical Communications, 2014 , vol. 50, # 40 p. 5248 - 5250 |
|
~76% 
chlorpropamide CAS#:94-20-2 |
| Literature: Mizuno, Takumi; Kino, Takanobu; Ito, Takatoshi; Miyata, Toshiyuki Synthetic Communications, 2000 , vol. 30, # 17 p. 3081 - 3089 |
|
~% 
chlorpropamide CAS#:94-20-2 |
| Literature: US4062889 A1, ; |
| Precursor 4 | |
|---|---|
| DownStream 8 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Sulfa drugs inhibit sepiapterin reduction and chemical redox cycling by sepiapterin reductase.
J. Pharmacol. Exp. Ther. 352(3) , 529-40, (2015) Sepiapterin reductase (SPR) catalyzes the reduction of sepiapterin to dihydrobiopterin (BH2), the precursor for tetrahydrobiopterin (BH4), a cofactor critical for nitric oxide biosynthesis and alkylgl... |
|
|
Evaluation of the in vitro/in vivo potential of five berries (bilberry, blueberry, cranberry, elderberry, and raspberry ketones) commonly used as herbal supplements to inhibit uridine diphospho-glucuronosyltransferase.
Food Chem. Toxicol. 72 , 13-9, (2014) In this study, we evaluated inhibitory potentials of popularly-consumed berries (bilberry, blueberry, cranberry, elderberry, and raspberry ketones) as herbal supplements on UGT1A1, UGT1A4, UGT1A6, UGT... |
|
|
Evaluation of thein vitro/in vivodrug interaction potential of BST204, a purified dry extract of ginseng, and its four bioactive ginsenosides through cytochrome P450 inhibition/induction and UDP-glucuronosyltransferase inhibition
Food Chem. Toxicol. 68 , 117-27, (2014) • BST204 is a purified dry extract of ginseng containing high amounts of Rh2 and Rg3. • BST204 had only weak inhibitory effects on nine CYPs and five UGTs. • It is unlikely that BST204 alter pharmacok... |
| Chloropropamide |
| Benzenesulfonamide, 4-chloro-N-((propylamino)carbonyl)- |
| chlorpropamide |
| Benzenesulfonamide, 4-chloro-N-[(Z)-hydroxy(propylimino)methyl]- |
| EINECS 202-314-5 |
| 4-chloro-N-(propylaminocarbonyl)benzenesulfonamide |
| N-[(4-Chlorophenyl)sulfonyl]-N'-propylcarbamimidic acid |
| MFCD00079004 |
| Benzenesulfonamide, 4-chloro-N-[(propylamino)carbonyl]- |
| clorpropamida [INN_es] |
| 1-(4-chlorophenyl)sulfonyl-3-propylurea |
| 4-Chloro-N-(propylcarbamoyl)benzenesulfonamide |
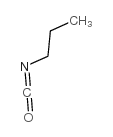
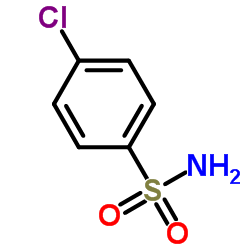
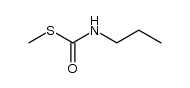
 CAS#:627-06-5
CAS#:627-06-5 CAS#:623-12-1
CAS#:623-12-1 CAS#:2050-68-2
CAS#:2050-68-2 CAS#:108-90-7
CAS#:108-90-7 CAS#:2051-62-9
CAS#:2051-62-9 CAS#:71-43-2
CAS#:71-43-2 CAS#:5769-15-3
CAS#:5769-15-3