| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
3-Maleimidopropionic acid
CAS:7423-55-4 |
|
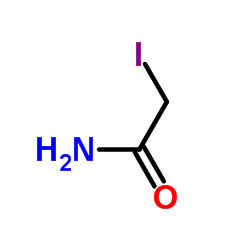 |
Iodoacetamide
CAS:144-48-9 |
|
 |
L-cysteine
CAS:52-90-4 |
|
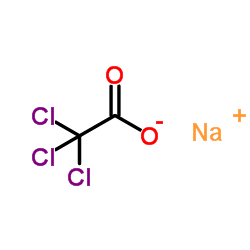 |
Sodium TCA
CAS:650-51-1 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
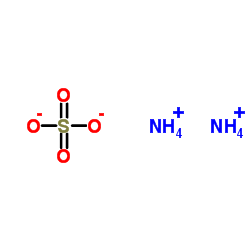 |
ammonium sulphate
CAS:7783-20-2 |
|
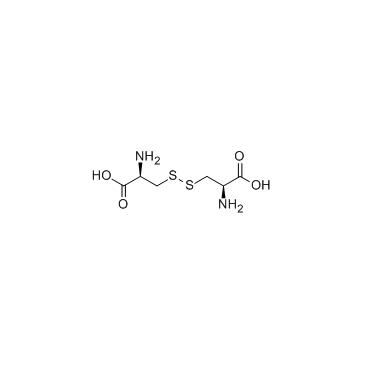 |
L-Cystine
CAS:56-89-3 |
|
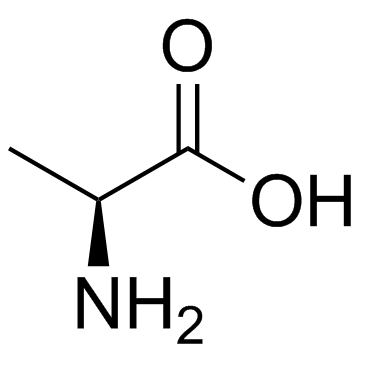 |
L-alanine
CAS:56-41-7 |
|
 |
NAD+
CAS:53-84-9 |