Iodoacetamide
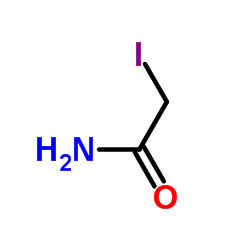
Iodoacetamide structure
|
Common Name | Iodoacetamide | ||
|---|---|---|---|---|
| CAS Number | 144-48-9 | Molecular Weight | 184.964 | |
| Density | 2.3±0.1 g/cm3 | Boiling Point | 297.1±23.0 °C at 760 mmHg | |
| Molecular Formula | C2H4INO | Melting Point | 92-95 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 133.5±22.6 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of Iodoacetamide2-Iodoacetamide (Iodoacetamide), an alkylating agent, is a commonly used agent for alkylation of cysteine during sample preparation for proteomics[1][2]. |
| Name | 2-Iodoacetamide |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Iodoacetamide (Iodoacetamide), an alkylating agent, is a commonly used agent for alkylation of cysteine during sample preparation for proteomics[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | 2-Iodoacetamide (2.0-10 mg; drinking; daily; 20-93 days) feeding always produces chronic deep ulcers in the secretory part of the rat stomach[2]. Animal Model: Sprague-Dawley rats[2] Dosage: 2.0-10 mg Administration: Drinking water, daily, for periods of 20 to 93 days Result: Produced gastritis which was further complicated by chronic ulceration in many of the test rats. |
| References |
[2]. J J LALICH. Iodoacetamide induced gastric ulcers in rats. Proc Soc Exp Biol Med. 1962 Apr;109:905-8. |
| Density | 2.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 297.1±23.0 °C at 760 mmHg |
| Melting Point | 92-95 °C(lit.) |
| Molecular Formula | C2H4INO |
| Molecular Weight | 184.964 |
| Flash Point | 133.5±22.6 °C |
| Exact Mass | 184.933746 |
| PSA | 43.09000 |
| LogP | -0.19 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.602 |
| Storage condition | 2-8°C |
| Stability | Stable, but light sensitive. Incompatible with strong oxidizing agents, strong bases, reducing agents, acids. |
| Water Solubility | slightly soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H317-H334-H413 |
| Precautionary Statements | P261-P280-P301 + P310-P342 + P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25;R36/37/38;R43 |
| Safety Phrases | S22-S36/37-S45-S37/39-S26-S24 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | AC4200000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2924199090 |
|
~66% 
Iodoacetamide CAS#:144-48-9 |
| Literature: Molecular Probes, Inc. Patent: US5274113 A1, 1993 ; |
|
~% 
Iodoacetamide CAS#:144-48-9 |
| Literature: Zeitschrift fuer Physikalische Chemie, Abteilung A: Chemische Thermodynamik, Kinetik, Elektrochemie, Eigenschaftslehre, , vol. 157, p. 249,254 Zeitschrift fuer Physikalische Chemie, Abteilung A: Chemische Thermodynamik, Kinetik, Elektrochemie, Eigenschaftslehre, , vol. 163, p. 38 |
|
~% 
Iodoacetamide CAS#:144-48-9 |
| Literature: Synthesis, , p. 574 - 575 |
|
~% 
Iodoacetamide CAS#:144-48-9 |
| Literature: Chemische Berichte, , vol. 41, p. 2117 Chemische Berichte, , vol. 42, p. 2056 |
| Precursor 4 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
A new enabling proteomics methodology to investigate membrane associated proteins from parasitic nematodes: case study using ivermectin resistant and ivermectin susceptible isolates of Caenorhabditis elegans and Haemonchus contortus.
Vet. Parasitol. 207(3-4) , 266-75, (2015) The mechanisms involved in anthelmintic resistance (AR) are complex but a greater understanding of AR management is essential for effective and sustainable control of parasitic helminth worms in lives... |
|
|
Rad23 interaction with the proteasome is regulated by phosphorylation of its ubiquitin-like (UbL) domain.
J. Mol. Biol. 426(24) , 4049-60, (2014) Rad23 was identified as a DNA repair protein, although a role in protein degradation has been described. The protein degradation function of Rad23 contributes to cell cycle progression, stress respons... |
|
|
Proteomic analysis of nasal epithelial cells from cystic fibrosis patients.
PLoS ONE 9(9) , e108671, (2014) The pathophysiology of cystic fibrosis (CF) lung disease remains incompletely understood. New explanations for the pathogenesis of CF lung disease may be discovered by studying the patterns of protein... |
| MFCD00008028 |
| carbamoylmethyl iodide |
| 2-Iodo-acetamide |
| USAF D-1 |
| Monoiodoacetamide |
| 2-iodoacetamido |
| Acetamide, 2-iodo- |
| Iodoacetamide |
| ACETAMIDE,2-IODO |
| 2-Iodoacetamide |
| Surauto |
| EINECS 205-630-1 |


![3,3-dimethyl-5'-oxo-1-ethyl-1,3-dihydrospiro[2H-indole-2,2'-pyrrolidine] structure](https://image.chemsrc.com/caspic/487/122275-78-9.png) CAS#:122275-78-9
CAS#:122275-78-9 CAS#:14134-81-7
CAS#:14134-81-7 CAS#:123-93-3
CAS#:123-93-3 CAS#:505-73-7
CAS#:505-73-7 CAS#:10034-85-2
CAS#:10034-85-2 CAS#:64-19-7
CAS#:64-19-7 CAS#:7553-56-2
CAS#:7553-56-2 CAS#:60-35-5
CAS#:60-35-5 CAS#:24466-68-0
CAS#:24466-68-0 CAS#:25077-26-3
CAS#:25077-26-3
