Stiripentol
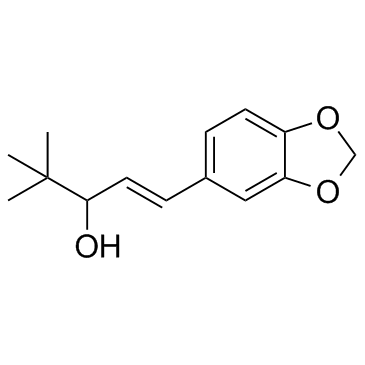
Stiripentol structure
|
Common Name | Stiripentol | ||
|---|---|---|---|---|
| CAS Number | 49763-96-4 | Molecular Weight | 234.291 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 365.4±11.0 °C at 760 mmHg | |
| Molecular Formula | C14H18O3 | Melting Point | 73-74ºC | |
| MSDS | Chinese USA | Flash Point | 174.8±19.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
The efficacy of bromides, stiripentol and levetiracetam in two patients with malignant migrating partial seizures in infancy.
Epileptic Disord. 13(1) , 22-6, (2011) The syndrome of malignant migrating partial seizures in infancy is a devastating, age-specific, epileptic encephalopathy, which still presents an aetiological, pathophysiological and therapeutic problem. In this study, we present two patients who were diagnos... |
|
|
Stiripentol in refractory status epilepticus.
Epilepsia 54 Suppl 6 , 103-5, (2013) Benzodiazepines (BZDs), which enhance γ-aminobutyric acid (GABAA ) receptor-mediated inhibition, are the first-line therapy for treatment of status epilepticus (SE). However, pharmacoresistance to BZDs develops rapidly after SE initiation. This is due to an a... |
|
|
[Clinical and genetic diagnosis of Dravet syndrome: report of 20 cases].
Ideggyogy. Sz. 61(11-12) , 402-8, (2008) Severe myoclonic epilepsy in infancy (SMEI; Dravet's syndrome) is a malignant epilepsy syndrome characterized by prolonged febrile hemiconvulsions or generalized seizures starting in the first year of life. Later on myoclonic, atypical absence, and complex pa... |
|
|
[Therapeutic drug monitoring of stiripentol].
Therapie. 67(2) , 157-60, (2012) Stiripentol is a third generation antiepileptic, marketed since 2007 under the name of Diacomit(®). It is indicated, always in combination, in the treatment of severe myoclonic epilepsy in infancy or Dravet syndrome. Its pharmacokinetics is not linear. It is ... |
|
|
Stiripentol for focal refractory epilepsy.
Cochrane Database Syst. Rev. 1 , CD009887, (2014) Nearly 30%of people with epilepsy do not have their seizures controlled with current treatments. Stiripentol is a new antiepileptic drug(AED) developed in France and recently approved by the European Medicines Agency (EMA) for the treatment of Dravet syndrome... |
|
|
Acute hepatic injury in a child with Dravet syndrome: no protective effect of stiripentol.
Seizure 17(5) , 477-8; author reply 479, (2008)
|
|
|
Stiripentol.
Epilepsy Res. Suppl. 3 , 153-6, (1991)
|
|
|
Ketogenic diet also benefits Dravet syndrome patients receiving stiripentol: a prospective pilot study.
Epilepsia 52(7) , e54-7, (2011) We aimed to test the efficacy of ketogenic diet (KD) in patients with Dravet syndrome (DS) not satisfactorily controlled by antiepileptic drugs (AEDs). We included prospectively 15 DS patients aged >3 years with partial response to AEDs including stiripentol.... |
|
|
Efficacy of stiripentol in hyperthermia-induced seizures in a mouse model of Dravet syndrome.
Epilepsia 53(7) , 1140-5, (2012) We previously reported a mutant mouse carrying a severe myoclonic epilepsy in infancy (SMEI) mutation in Scn1a. In this study, we examined the susceptibility to hyperthermia-induced seizures of heterozygous Scn1a mutant mice (Scn1a(RX/+)) and wild-type (Scn1a... |
|
|
Interactions between modulators of the GABA(A) receptor: Stiripentol and benzodiazepines.
Eur. J. Pharmacol. 654(2) , 160-5, (2011) Many patients with refractory epilepsy are treated with polytherapy, and nearly 15% of epilepsy patients receive two or more anti-convulsant agents. The anti-convulsant stiripentol is used as an add-on treatment for the childhood epilepsy syndrome known as se... |