[Therapeutic drug monitoring of stiripentol].
Marie-Clémence Verdier, Olivier Tribut, Danièle Bentué-Ferrer
Index: Therapie. 67(2) , 157-60, (2012)
Full Text: HTML
Abstract
Stiripentol is a third generation antiepileptic, marketed since 2007 under the name of Diacomit(®). It is indicated, always in combination, in the treatment of severe myoclonic epilepsy in infancy or Dravet syndrome. Its pharmacokinetics is not linear. It is a potent inhibitor of CYP3A4, 1A2 and 2C19 and increases the plasma concentrations of many other antiepileptic drugs. Without this being considered as a validated therapeutic range, the trough plasma concentrations at steady-state, corresponding to the usual doses are between 10 and 15 mg/L. The concentration-efficacy relationship is not established, but there is some evidence for a concentration-related toxicity. However, because of its non-linear kinetics, stiripentol should be a good candidate for therapeutic drug monitoring (TDM). Nonetheless, the current level of evidence for the advantage of TDM is "remains to be estimated".© 2012 Société Française de Pharmacologie et de Thérapeutique.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
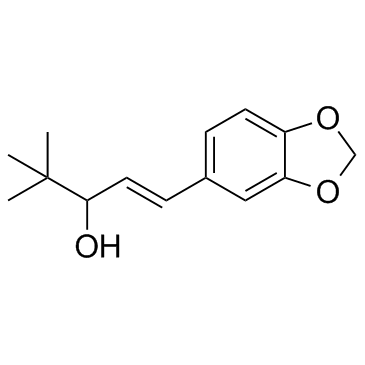 |
Stiripentol
CAS:49763-96-4 |
C14H18O3 |
|
The efficacy of bromides, stiripentol and levetiracetam in t...
2011-03-01 [Epileptic Disord. 13(1) , 22-6, (2011)] |
|
Stiripentol in refractory status epilepticus.
2013-09-01 [Epilepsia 54 Suppl 6 , 103-5, (2013)] |
|
[Clinical and genetic diagnosis of Dravet syndrome: report o...
2008-11-30 [Ideggyogy. Sz. 61(11-12) , 402-8, (2008)] |
|
Stiripentol for focal refractory epilepsy.
2014-01-01 [Cochrane Database Syst. Rev. 1 , CD009887, (2014)] |
|
Acute hepatic injury in a child with Dravet syndrome: no pro...
2008-07-01 [Seizure 17(5) , 477-8; author reply 479, (2008)] |