3-Methyloxindole
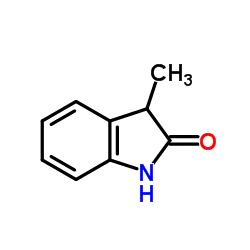
3-Methyloxindole structure
|
Common Name | 3-Methyloxindole | ||
|---|---|---|---|---|
| CAS Number | 1504-06-9 | Molecular Weight | 147.174 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 279.3±29.0 °C at 760 mmHg | |
| Molecular Formula | C9H9NO | Melting Point | 117-121ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 157.9±9.2 °C | |
|
Direct asymmetric anti-Mannich-type reactions catalyzed by cinchona alkaloid derivatives.
Chirality 26(12) , 801-5, (2014) A series of cinchona alkaloid derivatives were used to catalyze the asymmetric anti-Mannich-type reaction of 3-methyl-2-oxindole with N-tosyl aryl aldimines. The resulting anti-3,3-disubstituted 2-oxindole products were obtained in good yields (up to 92%) wit... |
|
|
Metabolism of 3-methylindole by vaccinia-expressed P450 enzymes: correlation of 3-methyleneindolenine formation and protein-binding.
J. Pharmacol. Exp. Ther. 276(1) , 21-9, (1996) The toxicity of 3-methylindole (3 MI), a selective pneumotoxin, is dependent upon cytochrome P450-mediated bioactivation 3. Using vaccinia-expressed P450 enzymes, the metabolites of radiolabeled 3 MI produced by 14 individual P450s were identified and quantif... |
|
|
Unbiased high-throughput screening of reactive metabolites on the linear ion trap mass spectrometer using polarity switch and mass tag triggered data-dependent acquisition.
Anal. Chem. 80(16) , 6410-22, (2008) Constant neutral loss (CNL) and precursor ion (PI) scan have been widely used for the in vitro screening of glutathione conjugates derived from reactive metabolites, but these two methods are only applicable to triple quadrupole or hybrid triple quadrupole ma... |
|
|
Metabolism and pneumotoxicity of 3-methyloxindole, indole-3-carbinol, and 3-methylindole in goats.
Am. J. Vet. Res. 43(8) , 1418-23, (1982)
|
|
|
Porcine CYP2A19, CYP2E1 and CYP1A2 forms are responsible for skatole biotransformation in the reconstituted system.
Neuro Endocrinol. Lett. 30 Suppl 1 , 36-40, (2009) To study the contribution of individual purified porcine CYP1A2, 2E1 and 2A19 enzymes to the biotransformation of skatole.Individual porcine and human enzymes (CYP1A2, 2E1 or 2A6/19) were used to study their potential involvement in skatole metabolism. Furthe... |
|
|
Facile and Efficient Enantioselective Hydroxyamination Reaction: Synthesis of 3-Hydroxyamino-2-Oxindoles Using Nitrosoarenes. Shen K, et al.
Angew. Chem. Weinheim Bergstr. Ger. 123(20) , 4780-4784, (2011)
|