Metabolism of 3-methylindole by vaccinia-expressed P450 enzymes: correlation of 3-methyleneindolenine formation and protein-binding.
J Thornton-Manning, M L Appleton, F J Gonzalez, G S Yost
Index: J. Pharmacol. Exp. Ther. 276(1) , 21-9, (1996)
Full Text: HTML
Abstract
The toxicity of 3-methylindole (3 MI), a selective pneumotoxin, is dependent upon cytochrome P450-mediated bioactivation 3. Using vaccinia-expressed P450 enzymes, the metabolites of radiolabeled 3 MI produced by 14 individual P450s were identified and quantified by high performance liquid chromatography. Indole-3-carbinol was produced from incubations of 3 MI with only four enzymes. Although 3-methyloxindole was a product of several P450s, human 1A2 was most efficient in producing this metabolite. The toxic intermediate of 3 MI is believed to be a reactive methylene imine, 3-methyleneindolenine. In this study, this intermediate was detected as its mercapturate adduct, when N-acetylcysteine was added to the incubations. 3-Methyleneindolenine was produced by CYP2A6 at a rate of 50.9 +/- 8.9 pmol/mg protein/hr and by CYP2F1 at a rate of 205.7 +/- 12.5 pmol/mg/hr. The mouse 1a-2 and rabbit 4B1 enzymes produced the reactive intermediate in amounts that exceeded that of the human 2F1 enzyme by 1.4-fold and 1.9-fold, respectively. The toxicity of 3 MI is believed to be due to covalent binding of a P450-generated intermediate to critical pulmonary proteins. Comparison of covalent binding studies to the formation of the metabolites revealed a strong correlation between the amount of the 3 MI adduct detected and covalent binding. This study showed that the methylene imine electrophile is produced by only a few P450 enzymes and is the metabolite responsible for the covalent binding and presumably, the toxicity of 3 MI. Remarkable product preferences between the desaturation pathway to form the methyleneindolenine by CYP2F1 and the ring epoxidation pathway to form the oxindole by CYP1A2, were observed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
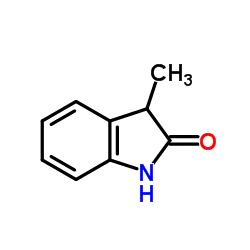 |
3-Methyloxindole
CAS:1504-06-9 |
C9H9NO |
|
Direct asymmetric anti-Mannich-type reactions catalyzed by c...
2014-12-01 [Chirality 26(12) , 801-5, (2014)] |
|
Unbiased high-throughput screening of reactive metabolites o...
2008-08-15 [Anal. Chem. 80(16) , 6410-22, (2008)] |
|
Metabolism and pneumotoxicity of 3-methyloxindole, indole-3-...
1982-08-01 [Am. J. Vet. Res. 43(8) , 1418-23, (1982)] |
|
Porcine CYP2A19, CYP2E1 and CYP1A2 forms are responsible for...
2009-01-01 [Neuro Endocrinol. Lett. 30 Suppl 1 , 36-40, (2009)] |
|
Facile and Efficient Enantioselective Hydroxyamination React...
[Angew. Chem. Weinheim Bergstr. Ger. 123(20) , 4780-4784, (2011)] |