2-Chloro-2-propen-1-ol
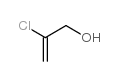
2-Chloro-2-propen-1-ol structure
|
Common Name | 2-Chloro-2-propen-1-ol | ||
|---|---|---|---|---|
| CAS Number | 5976-47-6 | Molecular Weight | 92.52420 | |
| Density | 1.162 g/mL at 25ºC(lit.) | Boiling Point | 133-134ºC(lit.) | |
| Molecular Formula | C3H5ClO | Melting Point | N/A | |
| MSDS | USA | Flash Point | 130 °F | |
| Symbol |


GHS02, GHS07 |
Signal Word | Warning | |
|
Kinetic studies of the homogeneous abiotic reactions of several chlorinated aliphatic compounds in aqueous solution. Pagan M, et al.
Appl. Geochem. 13(6) , 779-85, (1998)
|
|
|
Derivatives of phosphorus acids and 2-chloro-2-propen-1-ol. Arbuzov BA, et al.
Russ. Chem. Bull. 16(6) , 1233-38, (1967)
|
|
|
Asymmetric synthesis of conformationally constrained fingolimod analogues--discovery of an orally active sphingosine 1-phosphate receptor type-1 agonist and receptor type-3 antagonist.
J. Med. Chem. 50(25) , 6428-35, (2007) Compound 1 (FTY720, Fingolimod) represents a new generation of immunosuppressant that modulates lymphocyte trafficking by interacting with the S1P(1) receptor. Compound 1 also provides a template molecule for studying the molecular biology of S1P receptors an... |
|
|
Photofragment imaging study of the CH2CCH2OH radical intermediate of the OH+allene reaction.
J. Chem. Phys. 127(15) , 154316, (2007) These velocity map imaging experiments characterize the photolytic generation of one of the two radical intermediates formed when OH reacts via an addition mechanism with allene. The CH2CCH2OH radical intermediate is generated photolytically from the photodis... |
|
|
Degradation of 2-chloroallylalcohol by a Pseudomonas sp.
Appl. Environ. Microbiol. 59(2) , 528-35, (1993) Three Pseudomonas strains capable of utilizing 2-chloroallylalcohol (2-chloropropenol) as the sole carbon source for growth were isolated from soil. The fastest growth was observed with strain JD2, with a generation time of 3.6 h. Degradation of 2-chloroallyl... |