Photofragment imaging study of the CH2CCH2OH radical intermediate of the OH+allene reaction.
Arjun S Raman, M Justine Bell, Kai-Chung Lau, Laurie J Butler
Index: J. Chem. Phys. 127(15) , 154316, (2007)
Full Text: HTML
Abstract
These velocity map imaging experiments characterize the photolytic generation of one of the two radical intermediates formed when OH reacts via an addition mechanism with allene. The CH2CCH2OH radical intermediate is generated photolytically from the photodissociation of 2-chloro-2-propen-1-ol at 193 nm. Detecting the Cl atoms using [2+1] resonance-enhanced multiphoton ionization evidences an isotropic angular distribution for the Cl+CH2CCH2OH photofragments, a spin-orbit branching ratio for Cl(2P1/2):Cl(2P3/2) of 0.28, and a bimodal recoil kinetic energy distribution. Conservation of momentum and energy allows us to determine from this data the internal energy distribution of the nascent CH2CCH2OH radical cofragment. To assess the possible subsequent decomposition pathways of this highly vibrationally excited radical intermediate, we include electronic structure calculations at the G3//B3LYP level of theory. They predict the isomerization and dissociation transition states en route from the initial CH2CCH2OH radical intermediate to the three most important product channels for the OH+allene reaction expected from this radical intermediate: formaldehyde+C2H3, H+acrolein, and ethene+CHO. We also calculate the intermediates and transition states en route from the other radical adduct, formed by addition of the OH to the center carbon of allene, to the ketene+CH3 product channel. We compare our results to a previous theoretical study of the O+allyl reaction conducted at the CBS-QB3 level of theory, as the two reactions include several common intermediates.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
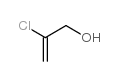 |
2-Chloro-2-propen-1-ol
CAS:5976-47-6 |
C3H5ClO |
|
Kinetic studies of the homogeneous abiotic reactions of seve...
[Appl. Geochem. 13(6) , 779-85, (1998)] |
|
Derivatives of phosphorus acids and 2-chloro-2-propen-1-ol. ...
[Russ. Chem. Bull. 16(6) , 1233-38, (1967)] |
|
Asymmetric synthesis of conformationally constrained fingoli...
2007-12-13 [J. Med. Chem. 50(25) , 6428-35, (2007)] |
|
Degradation of 2-chloroallylalcohol by a Pseudomonas sp.
1993-02-01 [Appl. Environ. Microbiol. 59(2) , 528-35, (1993)] |