Benzyl nicotinate (JAN)
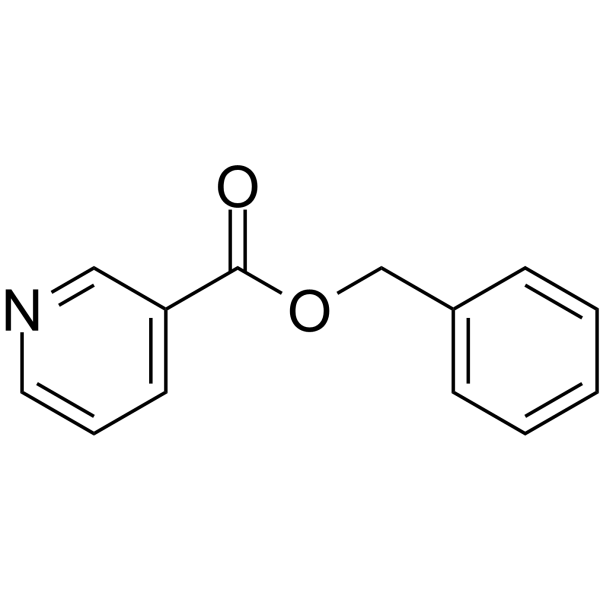
Benzyl nicotinate (JAN) structure
|
Common Name | Benzyl nicotinate (JAN) | ||
|---|---|---|---|---|
| CAS Number | 94-44-0 | Molecular Weight | 213.232 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 334.1±17.0 °C at 760 mmHg | |
| Molecular Formula | C13H11NO2 | Melting Point | 24 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 155.8±20.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Effects of water activity and low molecular weight humectants on skin permeability and hydration dynamics - a double-blind, randomized and controlled study.
Int. J. Cosmet. Sci. 36(5) , 412-8, (2014) The mammalian skin is a barrier that effectively separates the water-rich interior of the body from the normally dryer exterior. Changes in the external conditions, for example ambient humidity, have been shown to affect the skin barrier properties. The prime... |
|
|
[On interactions of drugs on and in the skin/comparative studies on the mutual influences of ethyleneglycol-monosalicylate and benzylnicotinate (author's transl)].
Derm. Beruf Umwelt. 29(6) , 161-7, (1981) Three radioactively marked substances (on the basis of a carbopol gel, 80% water - isopropanol) were analyzed comparatively in regard to the penetration of ethyleneglycolmonosalicylate and benzylnicotinate to intact and impaired human skin. The objective of t... |
|
|
Kinetics of blood flow after topical application of benzyl nicotinate on different anatomic sites.
Arch. Dermatol. Res. 298(6) , 291-300, (2006) Cutaneous characteristics, e.g., thickness of the SC and density of follicles, affect the penetration of topically applied substances. In the present study, the penetration of benzyl nicotinate, causing a vasodilation, was studied on three anatomic sites (for... |
|
|
Skin oxygenation after topical application of liposome-entrapped benzyl nicotinate as measured by EPR oximetry in vivo: influence of composition and size.
AAPS PharmSci 5(1) , E2, (2003) New and improved drug delivery systems are the important subject of much scientific research. The development of formulations that increase skin oxygenation and of methods for measuring oxygen levels in skin are important for dealing with healing processes af... |
|
|
Estimation of serum protein binding of compounds metabolized in serum using matrix inhibition.
Biopharm. Drug Dispos. 29(5) , 308-10, (2008) It is difficult to evaluate the serum protein binding of compounds that are metabolized in rat serum, even when using ultrafiltration. Protein binding was estimated using matrix inhibition, a method that uses the change in metabolic velocity achieved by chang... |
|
|
Blood flow enhancement in skin or oral mucosa after the topical application of liposome entrapped rubifacient as measured by EPR oximetry in vivo: the influence of size and liposome composition.
Cell Mol. Biol. Lett. 7(2) , 256-8, (2002)
|
|
|
Enhancement of bioavailability by lowering of fat content in topical formulations.
Br. J. Dermatol. 160(3) , 552-6, (2009) The cosmetic properties of topical formulations are important parameters for the adherence to treatment, where modern oil-in-water emulsions are considered more acceptable compared with ointments. After application of an emulsion to the skin, the concentratio... |
|
|
Effect of benzyl nicotinate on the percutaneous absorption of dexamethasone in the rat.
Eur. J. Drug Metab. Pharmacokinet. 5(1) , 25-8, (1980) The effect of benzyl nicotinate (15 mg) on the percutaneous absorption of dexamethasone 0,1 mg (62,5 uCi)/6 cm2, in an ethanol/octanol vehicle has been investigated in the rat. Both the urinary excretion and blood concentration data showed that benzyl nicotin... |
|
|
Improved skin oxygenation after benzyl nicotinate application in different carriers as measured by EPR oximetry in vivo.
J. Control. Release 70(1-2) , 203-11, (2001) The development of formulations, which increase skin oxygenation and of methods for measuring oxygen levels in skin are important for dealing with processes affected by the level of oxygen, e.g., rate of healing and efficiency of radiation oncology. In this s... |
|
|
Rhodium-catalyzed enantioselective addition of boronic acids to N-benzylnicotinate salts.
J. Am. Chem. Soc. 133(9) , 2878-80, (2011) The highly enantioselective catalytic asymmetric addition of aryl and alkenylboronic acids to N-benzylnicotinate salt 1 is described. The dihydropyridine 2 reaction products can be converted to synthetically useful piperidines. Application of the methodology ... |