| Structure | Name/CAS No. | Articles |
|---|---|---|
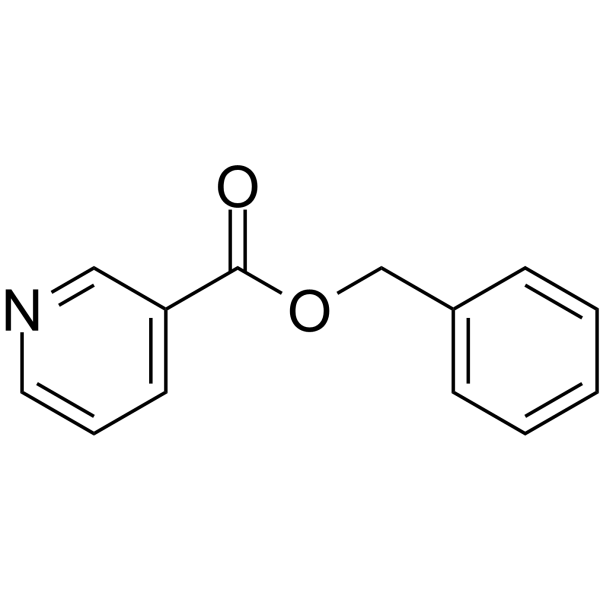 |
Benzyl nicotinate (JAN)
CAS:94-44-0 |
Nadeau Christian, Sara Aly, Kevin Belyk
Index: J. Am. Chem. Soc. 133(9) , 2878-80, (2011)
Full Text: HTML
The highly enantioselective catalytic asymmetric addition of aryl and alkenylboronic acids to N-benzylnicotinate salt 1 is described. The dihydropyridine 2 reaction products can be converted to synthetically useful piperidines. Application of the methodology to the preparation of enantioenriched quaternary chiral centers is also discussed.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Benzyl nicotinate (JAN)
CAS:94-44-0 |
C13H11NO2 |
|
Effects of water activity and low molecular weight humectant...
2014-10-01 [Int. J. Cosmet. Sci. 36(5) , 412-8, (2014)] |
|
[On interactions of drugs on and in the skin/comparative stu...
1981-01-01 [Derm. Beruf Umwelt. 29(6) , 161-7, (1981)] |
|
Kinetics of blood flow after topical application of benzyl n...
2006-11-01 [Arch. Dermatol. Res. 298(6) , 291-300, (2006)] |
|
Skin oxygenation after topical application of liposome-entra...
2003-01-01 [AAPS PharmSci 5(1) , E2, (2003)] |
|
Estimation of serum protein binding of compounds metabolized...
2008-07-01 [Biopharm. Drug Dispos. 29(5) , 308-10, (2008)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved