Bis(2,2,2-trichloroethyl)Azodicarboxylate
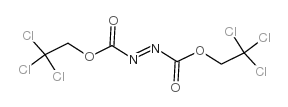
Bis(2,2,2-trichloroethyl)Azodicarboxylate structure
|
Common Name | Bis(2,2,2-trichloroethyl)Azodicarboxylate | ||
|---|---|---|---|---|
| CAS Number | 38857-88-4 | Molecular Weight | 380.82500 | |
| Density | 1.78g/cm3 | Boiling Point | 380.7ºC at 760 mmHg | |
| Molecular Formula | C6H4Cl6N2O4 | Melting Point | 109-111ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | 184.1ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Catalytic and enantioselective aza-ene and hetero-Diels-Alder reactions of alkenes and dienes with azodicarboxylates.
Org. Biomol. Chem. 3(12) , 2344-9, (2005) Lewis acids such as Cu(OTf)(2), Zn(OTf)(2), Yb(OTf)(3) and Nd(OTf)(3) catalyze the aza-ene reaction of alkenes with azodicarboxylates, giving the allylic amination adducts. The use of bis(2,2,2-trichloroethyl)azodicarboxylate as the amination reagent and Cu(O... |
|
|
Stereoselective formation of carbon-carbon bonds via SN2-displacement: synthesis of substituted cycloalkyl[b]indoles.
J. Org. Chem. 70(21) , 8385-94, (2005) A general asymmetric synthesis of substituted cycloalkyl[b]indoles has been accomplished. The key features of this approach are (1) the utilization of a Japp-Klingemann condensation/Fischer cyclization to prepare cycloalkyl[b]indolones, (2) the asymmetric bor... |
|
|
A convenient synthesis of N-acetyllactosamine derivatives from lactal.
Carbohydr. Res. 247 , 159-64, (1993) In a thermal inverse-type hetero-Diels-Alder reaction of O-silyl-protected lactal 1 and bis(2,2,2-trichloroethyl) azodicarboxylate (2), the dihydrooxadiazine derivative 3 was obtained in a very high yield; transesterification with benzyl alcohol furnished the... |
|
|
N. Boudreault, Y. Leblanc
Organic Synth. 74 , 241, (1997)
|
|
|
R.D. Little et al.
J. Am. Chem. Soc. 101 , 7129, (1979)
|
|
|
M. Kaname et al.
Tetrahedron Lett. 40 , 7993 , (1999)
|
|
|
Y. Leblanc, B.J. Fitzsimmons
Tetrahedron Lett. 30 , 2889, (1989)
|
|
|
Organic Synth. 61 , 17, (1983)
|
|
|
Para-directed amination of electron-rich arenes with bis (2, 2, 2-trichloroethyl) azodicarboxylate. Leblanc Y and Boudreault N.
J. Org. Chem. 60(13) , 4268-71, (1995)
|