6-Chloronicotinoyl chloride
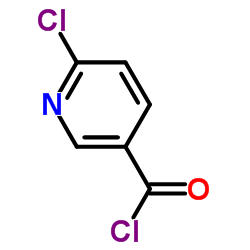
6-Chloronicotinoyl chloride structure
|
Common Name | 6-Chloronicotinoyl chloride | ||
|---|---|---|---|---|
| CAS Number | 66608-11-5 | Molecular Weight | 176.000 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 246.2±20.0 °C at 760 mmHg | |
| Molecular Formula | C6H3Cl2NO | Melting Point | 48-51ºC(lit.) | |
| MSDS | USA | Flash Point | 102.7±21.8 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Substituted 1,3-dipropylxanthines as irreversible antagonists of A1 adenosine receptors.
J. Med. Chem. 37(17) , 2704-12, (1994) This report describes the synthesis of 29 xanthines containing a chemoreactive chloroaryl, beta-chloroethylamino, alpha,beta-unsaturated carbonyl, bromoacetyl, 3-(fluorosulfonyl)benzoyl, or 4-(fluorosulfonyl)benzoyl group as part of an exocyclic 1-, 3-, or 8-... |
|
|
Asymmetric chloronicotinyl insecticide, 1-[1-(6-chloro-3-pyridyl)ethyl]-2-nitroiminoimidazolidine: preparation, resolution and biological activities toward insects and their nerve preparations.
Biosci. Biotechnol. Biochem. 67(5) , 980-8, (2003) The asymmetric chloronicotinyl insecticide, 1-[1-(6-chloro-3-pyridyl)ethyl]-2-nitroiminoimidazolidine, was prepared, and the absolute configurations of the enantiomers were determined by an X-ray analysis. The insecticidal activity against the housefly measur... |
|
|
[3H] imidacloprid: Synthesis of a candidate radioligand for the nicotinic acetylcholine receptor. Latli B and Casida JE.
J. Labelled Comp. Radiopharm. 31(8) , 609-613, (1992)
|
|
|
[6-chloro-3-pyridylmethyl-3H] neonicotinoids as high-affinity radioligands for the nicotinic acetylcholine receptor: Preparation using NaB3H4 and LiB3H4. Latli B, et al.
J. Labelled Comp. Radiopharm. 38(11)971-978 , 971-978, (1996)
|
|
|
Nicotinic acid crown ethers. Synthesis and structural characterization of polyethereal macrocyclic lactones from 6-chloronicotinic acid. Newkome GR, et al.
J. Org. Chem. 45(26) , 5423-5425, (1980)
|
|
|
Preparation of thermally stable, low dielectric constant, pyridine-based polyimide and related nanofoams. Aram E and Mehdipour-Ataei S.
J. Appl. Polym. Sci. 128(6) , 4387-4394, (2013)
|