Asymmetric chloronicotinyl insecticide, 1-[1-(6-chloro-3-pyridyl)ethyl]-2-nitroiminoimidazolidine: preparation, resolution and biological activities toward insects and their nerve preparations.
Shinzo Kagabu, Kazuhisa Kiriyama, Hisashi Nishiwaki, Yuko Kumamoto, Toshiji Tada, Keiichiro Nishimura
Index: Biosci. Biotechnol. Biochem. 67(5) , 980-8, (2003)
Full Text: HTML
Abstract
The asymmetric chloronicotinyl insecticide, 1-[1-(6-chloro-3-pyridyl)ethyl]-2-nitroiminoimidazolidine, was prepared, and the absolute configurations of the enantiomers were determined by an X-ray analysis. The insecticidal activity against the housefly measured with metabolic inhibitors showed the (S) enantiomer to be slightly more active than the (R) isomer. Electrophysiological measurements on the American cockroach central nerve cord showed the compounds to elicite the impulses and subsequently blocked them. The neuroblocking potency of the (S) isomer was 5.9 microM, while that of the (R) isomer was as high as 73 microM. The molar concentrations required for 50% inhibition of the specific binding of [3H]imidacloprid to the housefly head membrane preparation were respectively 0.19 microM and 0.95 microM for the (S) and (R) isomers. This enatioselectivity ratio was smaller than 35 for nicotine isomers but greater than 2 for epibatidine isomers.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
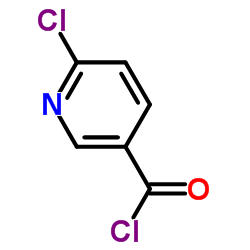 |
6-Chloronicotinoyl chloride
CAS:66608-11-5 |
C6H3Cl2NO |
|
Substituted 1,3-dipropylxanthines as irreversible antagonist...
1994-08-19 [J. Med. Chem. 37(17) , 2704-12, (1994)] |
|
[3H] imidacloprid: Synthesis of a candidate radio...
[J. Labelled Comp. Radiopharm. 31(8) , 609-613, (1992)] |
|
[6-chloro-3-pyridylmethyl-3H] neonicotinoids as high-...
[J. Labelled Comp. Radiopharm. 38(11)971-978 , 971-978, (1996)] |
|
Nicotinic acid crown ethers. Synthesis and structural charac...
[J. Org. Chem. 45(26) , 5423-5425, (1980)] |
|
Preparation of thermally stable, low dielectric constant, py...
[J. Appl. Polym. Sci. 128(6) , 4387-4394, (2013)] |