styrylboronic acid
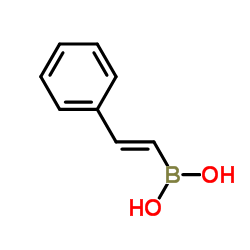
styrylboronic acid structure
|
Common Name | styrylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 6783-05-7 | Molecular Weight | 147.967 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 315.9±35.0 °C at 760 mmHg | |
| Molecular Formula | C8H9BO2 | Melting Point | 146-156 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 144.9±25.9 °C | |
|
Rh2(II)-catalyzed intramolecular aliphatic C-H bond amination reactions using aryl azides as the N-atom source.
J. Am. Chem. Soc. 17th ed., 134 , 7262-7265, (2012) Rhodium(II) dicarboxylate complexes were discovered to catalyze the intramolecular amination of unactivated primary, secondary, or tertiary aliphatic C-H bonds using aryl azides as the N-atom precursor. While a strong electron-withdrawing group on the nitroge... |
|
|
Copper-mediated sequential cyanation of aryl C-B and arene C-H bonds using ammonium iodide and DMF.
J. Am. Chem. Soc. 5th ed., 134 , 2528-2531, (2012) The cyanation of aromatic boronic acids, boronate esters, and borate salts was developed under copper-mediated oxidative conditions using ammonium iodide and DMF as the source of nitrogen and carbon atom of the cyano unit, respectively. The procedure was succ... |
|
|
Ambient temperature synthesis of high enantiopurity N-protected peptidyl ketones by peptidyl thiol ester-boronic acid cross-coupling.
J. Am. Chem. Soc. 129 , 1132, (2007) alpha-Amino acid thiol esters derived from N-protected mono-, di-, and tripeptides couple with aryl, pi-electron-rich heteroaryl, or alkenyl boronic acids in the presence of stoichiometric Cu(I) thiophene-2-carboxylate and catalytic Pd(2)(dba)(3)/triethylphos... |
|
|
Build/couple/pair strategy combining the Petasis 3-component reaction with Ru-catalyzed ring-closing metathesis and isomerization.
ACS Comb. Sci. 4th ed., 14 , 253-257, (2012) A "build/couple/pair" pathway for the systematic synthesis of structurally diverse small molecules is presented. The Petasis 3-component reaction was used to synthesize anti-amino alcohols displaying pairwise reactive combinations of alkene moieties. Upon tre... |
|
|
Iridium-catalyzed addition of aroyl chlorides and aliphatic acid chlorides to terminal alkynes.
J. Am. Chem. Soc. 2nd ed., 134 , 1268-1274, (2012) Iridium complexes show high catalytic activity in intermolecular additions of acid chlorides to terminal alkynes to afford valuable (Z)-β-chloro-α,β-unsaturated ketones. Ligands in the catalytic system play a crucial role in this reaction. An N-heterocyclic c... |
|
|
Rh(I)-catalyzed asymmetric 1,2-addition to α-diketones with chiral sulfur-alkene hybrid ligands.
Org. Lett. 2nd ed., 14 , 624-627, (2012) This paper describes a Rh(I)-catalyzed highly efficient and enantioselective 1,2-addition of arylboronic acids to α-diketones with the use of a simple sulfur-alkene hybrid ligand. With as low as a 0.1 mol % catalyst loading, a variety of optically active α-hy... |
|
|
Lewis acid promoted highly diastereoselective Petasis Borono-Mannich reaction: efficient synthesis of optically active β,γ-unsaturated α-amino acids.
Org. Lett. 8th ed., 14 , 2062-2065, (2012) An efficient and straightforward method for the preparation of highly enantiomerically enriched β,γ-unsaturated α-amino acid derivatives by a Lewis acid promoted diastereoselective Petasis reaction of vinylboronic acid, N-tert-butanesulfinamide, and glyoxylic... |
|
|
Palladium-catalyzed cascade cyclization of ynamides to azabicycles.
Chemistry 51th ed., 17 , 14366-14370, (2011) Cascade reactions: A modular assembly of azabicycles by using a cascade cyclization/Suzuki coupling/6π-electrocyclization of bromoenynamides is reported. The reaction offers a wide substituent scope on the bicyclic aminodiene products, which can be selectivel... |
|
|
Chem. Commun. (Camb.) , 1200-1201, (2004)
|
|
|
Ligand Effects on the Stereochemical Outcome of Suzuki-Miyaura Couplings Lu, G-P.; et al.
J. Org. Chem. 8th ed., 77 , 370-3703, (2012)
|