| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Methyl 4-boronobenzoate
CAS:99768-12-4 |
|
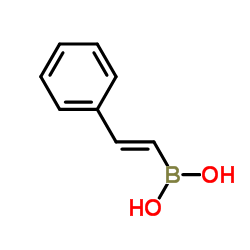 |
styrylboronic acid
CAS:6783-05-7 |