| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Methyl 4-boronobenzoate
CAS:99768-12-4 |
|
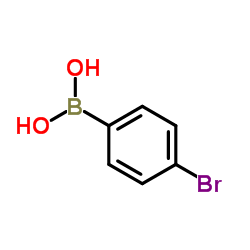 |
4-Bromophenylboronic Acid
CAS:5467-74-3 |
|
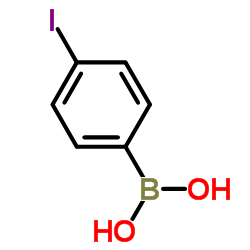 |
p-Iodobenzeneboronic acid
CAS:5122-99-6 |
|
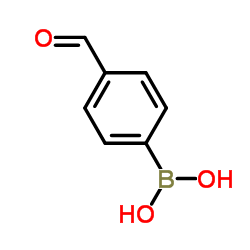 |
4-Formylphenylboronic acid
CAS:87199-17-5 |
|
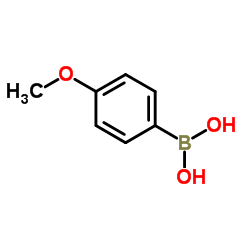 |
4-Methoxyphenylboronic acid
CAS:5720-07-0 |