p-nitrophenacyl bromide
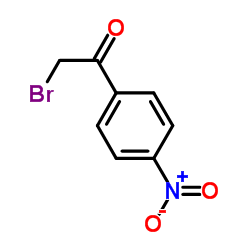
p-nitrophenacyl bromide structure
|
Common Name | p-nitrophenacyl bromide | ||
|---|---|---|---|---|
| CAS Number | 99-81-0 | Molecular Weight | 244.042 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 325.2±17.0 °C at 760 mmHg | |
| Molecular Formula | C8H6BrNO3 | Melting Point | 94-99 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 150.5±20.9 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
QSAR study and conformational analysis of 4-arylthiazolylhydrazones derived from 1-indanones with anti-Trypanosoma cruzi activity.
Eur. J. Pharm. Sci. 78 , 190-7, (2015) A set of 4-arylthiazolylhydrazones derived from 1-indanones (TZHs) previously synthesized and assayed against Trypanosoma cruzi, the causative agent of Chagas disease, were explored in terms of conformational analysis. We found that TZHs can adopt four minimu... |
|
|
Active site of bee venom phospholipase A2: the role of histidine-34, aspartate-64 and tyrosine-87.
Biochemistry 35(14) , 4591-601, (1996) In bee venom phospholipase A2, histidine-34 probably functions as a Brønsted base to deprotonate the attacking water. Aspartate-64 and tyrosine-87 form a hydrogen bonding network with histidine-34. We have prepared mutants at these positions and studied their... |
|
|
Chemical modification and inactivation of rat liver microsomal cytochrome P-450c by 2-bromo-4'-nitroacetophenone.
J. Biol. Chem. 261(25) , 11478-86, (1986) The alkylating agent 2-bromo-4'-nitroacetophenone (BrNAP) binds covalently to each of 10 isozymes of purified rat liver microsomal cytochrome P-450 (P-450a-P-450j) but substantially inhibits the catalytic activity of only cytochrome P-450c. Regardless of pH, ... |
|
|
[Amphoteric ion intermediates in the pyrazine series. II. The action of p-nitrophenacyl bromide on pyrazine].
Rev. Med. Chir. Soc. Med. Nat. Iasi. 94(1) , 157-60, (1990) Continuing to present the results of the investigations carried out on the amphoteric ions intermediates of the N-heteroatomic system with 2 natrium atoms in positions 1, 4, new pyrasine derivatives synthetized with p-nitro-phenacyl bromide are described. The... |
|
|
Use of an imperfect neutral diluent and outer vesicle layer scooting mode hydrolysis to analyze the interfacial kinetics, inhibition, and substrate preferences of bee venom phospholipase A2.
Biochemistry 36(13) , 3870-81, (1997) Interfacial catalytic constants for bee venom phospholipase A2 (bvPLA2) have been obtained for its action on vesicles of the anionic phospholipid 1,2-dimyristoylphosphatidylmethanol (DMPM) in the highly processive scooting mode. Spectroscopic measurements whi... |
|
|
Mechanism of inactivation of rat liver microsomal cytochrome P-450c by 2-bromo-4'-nitroacetophenone.
J. Biol. Chem. 261(25) , 11487-95, (1986) The mechanism by which 2-bromo-4'-nitroacetophenone (BrNAP) inactivates cytochrome P-450c, which involves alkylation primarily at Cys-292, is shown in the present study to involve an uncoupling of NADPH utilization and oxygen consumption from product formatio... |