(D-Ser(tBu)6,AzaGly10)-LHRH acetate salt
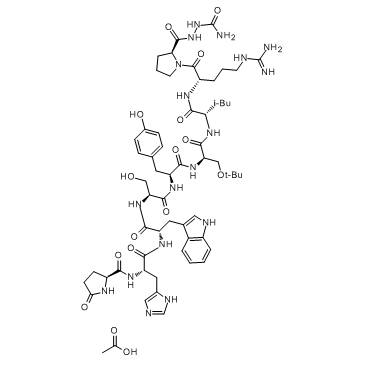
(D-Ser(tBu)6,AzaGly10)-LHRH acetate salt structure
|
Common Name | (D-Ser(tBu)6,AzaGly10)-LHRH acetate salt | ||
|---|---|---|---|---|
| CAS Number | 145781-92-6 | Molecular Weight | 1329.46 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 1695.5ºC at 760mmHg | |
| Molecular Formula | C61H88N18O16 | Melting Point | >190°C (dec.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS08 |
Signal Word | Danger | |
|
Stability of peptide drugs in the colon.
Eur. J. Pharm. Sci. 78 , 31-6, (2015) This study was the first to investigate the colonic stability of 17 peptide molecules (insulin, calcitonin, glucagon, secretin, somatostatin, desmopressin, oxytocin, Arg-vasopressin, octreotide, ciclosporin, leuprolide, nafarelin, buserelin, histrelin, [D-Ser... |
|
|
Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo.
Nanomedicine: Nanotechnology, Biology, and Medicine 11 , 1277-83, (2015) We report potent radiosensitization of prostate cancers in vitro and in vivo using goserelin-conjugated gold nanorods. Progressive receptor-mediated internalization of conjugated nanorods over time increases the radiation interaction cross-section of cells an... |
|
|
Toward oral delivery of biopharmaceuticals: an assessment of the gastrointestinal stability of 17 peptide drugs.
Mol. Pharm. 12(3) , 966-73, (2015) A major barrier to successful oral delivery of peptide and protein molecules is their inherent instability in the lumen of the gastrointestinal tract. The aim of this study was to determine the stability of 17 disparate peptide drugs (insulin, calcitonin, glu... |
|
|
Phase I, two-way, crossover study to demonstrate bioequivalence and to compare safety and tolerability of single-dose XM17 vs Gonal-f® in healthy women after follicle-stimulating hormone downregulation.
Reprod. Biol. Endocrinol. 13 , 130, (2015) XM17 is a recombinant human follicle-stimulating hormone (rhFSH) intended mainly for use in controlled ovarian hyperstimulation and the treatment of anovulation. The purpose of the current study was to establish bioequivalence, safety and tolerability of sing... |
|
|
Significance of baseline bone markers on disease progression and survival in hormone-sensitive prostate cancer with bone metastasis.
World J. Urol. 33 , 1263-8, (2015) This study evaluated the baseline patient characteristics associated with the time to biochemical progression and overall survival in patients who participated in a phase II trial on zoledronic acid combined with the initial androgen-deprivation therapy for t... |
|
|
Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin.
N. Engl. J. Med. 337(5) , 295-300, (1997) We conducted a randomized, prospective trial comparing external irradiation with external irradiation plus goserelin (an agonist analogue of gonadotropin-releasing hormone that reduces testosterone secretion) in patients with locally advanced prostate cancer.... |
|
|
Controlled release of therapeutic agents: slow delivery and cell encapsulation.
World J. Urol. 18(1) , 80-3, (2000) Some of the most promising systems for the controlled release of bioactive agents, i.e., peptides or hormones, involve the encapsulation or entrapment of hormones or peptides in biocompatible polymeric devices that enable their continuous release over prolong... |
|
|
Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy.
N. Engl. J. Med. 372(10) , 923-32, (2015) Ovarian failure is a common toxic effect of chemotherapy. Studies of the use of gonadotropin-releasing hormone (GnRH) agonists to protect ovarian function have shown mixed results and lack data on pregnancy outcomes.We randomly assigned 257 premenopausal wome... |
|
|
Injectable controlled release depots for large molecules.
J. Control. Release 190 , 240-53, (2014) Biodegradable, injectable depot formulations for long-term controlled drug release have improved therapy for a number of drug molecules and led to over a dozen highly successful pharmaceutical products. Until now, success has been limited to several small mol... |
|
|
The effects of androgen deprivation therapy with weight management on serum aP2 and adiponectin levels in prostate cancer patients.
Aging Male 18 , 72-6, (2015) Androgen deprivation therapy (ADT) for the treatment of prostate cancer (PCa) causes an increase in total body fat, leading to a net gain in body weight. Moreover, the use of the luteinizing hormone-releasing hormone agonists in ADT causes a decrease in serum... |