2,5-Hexanediol
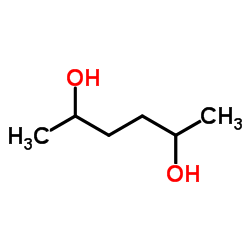
2,5-Hexanediol structure
|
Common Name | 2,5-Hexanediol | ||
|---|---|---|---|---|
| CAS Number | 2935-44-6 | Molecular Weight | 118.174 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 213.4±8.0 °C at 760 mmHg | |
| Molecular Formula | C6H14O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 101.7±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Pattern of neurotoxicity of n-hexane, methyl n-butyl ketone, 2,5-hexanediol, and 2,5-hexanedione alone and in combination with O-ethyl O-4-nitrophenyl phenylphosphonothioate in hens.
J. Toxicol. Environ. Health A 16(1) , 85-100, (1985) This investigation was designed to study the neurotoxicity produced in hens by the aliphatic hexacarbons n-hexane, methyl n-butyl ketone (MnBK), 2,5-hexanediol (2,5-HDOH), and 2,5-hexanedione (2,5-HD) following daily dermal application of each chemical alone ... |
|
|
The antimycotic activity in vitro of five diols.
Sabouraudia 18(4) , 287-93, (1980) The antimycotic activity of ethane-1,2-diol, propane-1,2-diol, butane-1,3-diol, pentane-1,5-diol, and hexane-2,5-diol in vitro against Pityrosporum orbiculare, Candida albicans, Trichophyton rubrum, T. mentagrophytes var. interdigitale and Epidermophyton floc... |
|
|
[Effects of 2,5-hexanediol, 2,4-pentanedione, acetone and 2-heptanone on the secretory responsiveness of the sweat glands in rats (author's transl)].
Sangyo. Igaku. 22(6) , 494-5, (1980)
|
|
|
Highly efficient synthesis of enantiopure diacetylated C(2)-symmetric diols by ruthenium- and enzyme-catalyzed dynamic kinetic asymmetric transformation (DYKAT).
Chemistry 12 , 6053, (2006) Highly efficient synthesis of enantiopure diacetates of 2,4-pentanediol and 2,5-hexanediol starting from commercially available mixtures of the diols (dl/meso approximately 1:1) has been realized by combining a fast ruthenium-catalyzed epimerization with an e... |
|
|
Recovery from 2,5-hexanediol intoxication of the retinotectal tract of the rat. An ultrastructural study.
Acta Neuropathol. 58(4) , 286-90, (1982) Rats were given 2,5-hexanediol, a metabolite of n-hexane, in the drinking water until they developed a marked degree of paresis over about 7 weeks and were then allowed to recover naturally. The time course and the manner of removal of the neurofilamentous ma... |
|
|
Diastereoselective synthesis of optically active (2 R,5 R)-hexanediol.
Appl. Microbiol. Biotechnol. 58(5) , 595-9, (2002) Diastereoselective reduction of diketones with Lactobacillus kefir DSM 20587 was examined. The reduction of both oxo-functions proceeded highly diastereoselectively. (2 R,5 R)-Hexanediol 3 was produced starting from (2,5)-hexanedione 1 in quantitative yields ... |
|
|
Cytochemical staining characteristics of peripheral nodes of Ranvier in hexacarbon intoxication.
J. Neurocytol. 12(3) , 459-73, (1983) Changes in the distribution of stainable gap substance and subaxolemmal density at peripheral nodes of Ranvier in hexacarbon-intoxicated rats have been studied by light and electron microscopy. Cupric ion binding to the nodal gap substance was seen in normal ... |
|
|
Interaction between neurotoxicities induced by organophosphorus and long-chain hexacarbon compounds.
Neurotoxicology 4(4) , 117-35, (1983)
|
|
|
Toxicological and metabolic studies of methyl n-butylketone, 2,5-hexanedione, and 2,5-hexanediol in male rats.
Ecotoxicol. Environ. Saf. 3(2) , 204-17, (1979)
|
|
|
Central nervous system effect of 2,5-hexane diol.
Neurotoxicology 6(3) , 53-9, (1985) Single exposure of 150, 300 and 600 mg/kg 2,5-hexanediol, (2,5 HD) a metabolite of n-hexane showed no significant effect on 3H-spiroperidol binding to corpus striatal membranes when compared with control animals, where as repeated exposure of 2,5-HD, 300 and ... |