Diastereoselective synthesis of optically active (2 R,5 R)-hexanediol.
J Haberland, A Kriegesmann, E Wolfram, W Hummel, A Liese
Index: Appl. Microbiol. Biotechnol. 58(5) , 595-9, (2002)
Full Text: HTML
Abstract
Diastereoselective reduction of diketones with Lactobacillus kefir DSM 20587 was examined. The reduction of both oxo-functions proceeded highly diastereoselectively. (2 R,5 R)-Hexanediol 3 was produced starting from (2,5)-hexanedione 1 in quantitative yields with enantiomeric excess >99% and diastereomeric excess >99%. The reaction conditions were optimized: maximum yield of (2 R,5 R)-hexanediol was reached at pH 6, 30 degrees C and with equal amounts of substrate and cosubstrate. The applicability of the system in fed-batch experiments was demonstrated. The feed specific biomass concentration required to reach maximal yield and selectivity in fed-batch mode was determined.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
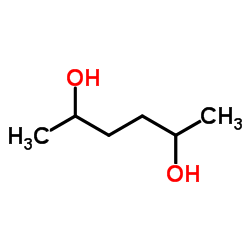 |
2,5-Hexanediol
CAS:2935-44-6 |
C6H14O2 |
|
Pattern of neurotoxicity of n-hexane, methyl n-butyl ketone,...
1985-01-01 [J. Toxicol. Environ. Health A 16(1) , 85-100, (1985)] |
|
The antimycotic activity in vitro of five diols.
1980-12-01 [Sabouraudia 18(4) , 287-93, (1980)] |
|
[Effects of 2,5-hexanediol, 2,4-pentanedione, acetone and 2-...
1980-11-01 [Sangyo. Igaku. 22(6) , 494-5, (1980)] |
|
Highly efficient synthesis of enantiopure diacetylated C(2)-...
2006-08-07 [Chemistry 12 , 6053, (2006)] |
|
Recovery from 2,5-hexanediol intoxication of the retinotecta...
1982-01-01 [Acta Neuropathol. 58(4) , 286-90, (1982)] |