3-Oxo-N-phenylbutanamide
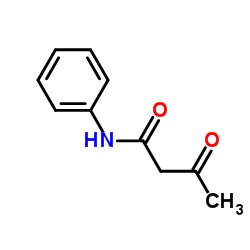
3-Oxo-N-phenylbutanamide structure
|
Common Name | 3-Oxo-N-phenylbutanamide | ||
|---|---|---|---|---|
| CAS Number | 102-01-2 | Molecular Weight | 177.200 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 329.0±44.0 °C at 760 mmHg | |
| Molecular Formula | C10H11NO2 | Melting Point | 83-88 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 152.8±28.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Kinetics and mechanisms of reactions between H2O2 and copper and copper oxides.
Dalton Trans. 44 , 16045-51, (2015) One of the main challenges for the nuclear power industry today is the disposal of spent nuclear fuel. One of the most developed methods for its long term storage is the Swedish KBS-3 concept where the spent fuel is sealed inside copper canisters and placed 5... |
|
|
Acetoacetanilides as masked isocyanates: facile and efficient synthesis of unsymmetrically substituted ureas.
Org. Lett. 12 , 4220-4223, (2010) A general and practical method for the preparation of unsymmetrically substituted ureas has been developed. By the reactions of acetoacetanilides with various amines including primary/secondary amines, a series of substituted aryl ureas were achieved in high ... |
|
|
Massive screening yields novel and selectiveTrypanosoma cruzitriosephosphate isomerase dimer-interface-irreversible inhibitors with anti-trypanosomal activity
Eur. J. Med. Chem. 45 , 5767-72, (2010) Triosephosphate isomerase from Trypanosoma cruzi (TcTIM), an enzyme in the glycolytic pathway that exhibits high catalytic rates of glyceraldehyde-3-phosphate- and dihydroxyacetone-phosphate-isomerization only in its dimeric form, was screened against an in-h... |
|
|
FeCl3⋅6H2O-catalyzed intermolecular-cascade cyclization of acetoacetanilide: aldehyde-tuned synthesis to valuable 2-pyridone analogues.
Chemistry 18(7) , 1905-9, (2012) The first ever breakthrough toward activation of β-ketoacetanilide and subsequent C-C and C-N bond-forming intermolecular-cascade cyclization processes is demonstrated by development of the unprecedented Lewis acid property of non-toxic FeCl(3)⋅6H(2)O. Aromat... |
|
|
Regiospecific β-lactam ring-opening/recyclization reactions of N-aryl-3-spirocyclic-β-lactams catalyzed by a Lewis-Brønsted acids combined superacid catalyst system: a new entry to 3-spirocyclicquinolin-4(1H)-ones.
Chem. Commun. (Camb.) 48(5) , 690-2, (2012) The regiospecific β-lactam ring-opening/recyclization reaction of N-aryl-3-spirocyclic-β-lactams, made by the one-pot cyclization reaction of acetoacetanilides, has been achieved for the first time using a Lewis-Brønsted acids combined superacid catalyst syst... |
|
|
Spectroscopic, and thermal studies of some new binuclear transition metal(II) complexes with hydrazone ligands containing acetoacetanilide and isoxazole.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 68(3) , 918-26, (2007) A new chelating ligand, 2-(2-(5-tert-butylisoxazol-3-yl)hydrazono)-N-(2,4-dimethylphenyl)-3-oxobutanamide (HL), and its four binuclear transition metal complexes, M(2)(L)(2) (micro-OCH(3))(2) [M=Ni(II), Co(II), Cu(II), Zn(II)], were synthesized using the proc... |
|
|
Syntheses of 1,3-imidazolin-2-ones and 1,3-imidazolin-2-thiones from new building blocks, gamma-aminoacetoacetanilides.
J. Comb. Chem. 10(6) , 803-6, (2008)
|
|
|
Thin-layer chromatographic separation of some sulpha drugs using acetoacetanilide as a coupling agent.
J. Chromatogr. A. 441(2) , 454-7, (1988)
|
|
|
Optical, elemental and structural analyses of acetoacetanilide single crystals for nonlinear optical applications.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 93 , 75-80, (2012) Single crystals of acetoacetanilide have been successfully grown by slow evaporation solution growth method at room temperature. The grown crystal belongs to orthorhombic crystal system having the lattice dimensions of a=8.686Å, b=11.104Å, c=19.232Å. Its crys... |
|
|
Crystal structures of azo pigments derived from acetoacetanilide Whitaker, A.
J. Soc. Dyers Colourists 104.7-8 , 294-300, (1988)
|