Chemical Communications
2012-01-18
Regiospecific β-lactam ring-opening/recyclization reactions of N-aryl-3-spirocyclic-β-lactams catalyzed by a Lewis-Brønsted acids combined superacid catalyst system: a new entry to 3-spirocyclicquinolin-4(1H)-ones.
Yinqiao Hu, Xiaolan Fu, Badru-Deen Barry, Xihe Bi, Dewen Dong
Index: Chem. Commun. (Camb.) 48(5) , 690-2, (2012)
Full Text: HTML
Abstract
The regiospecific β-lactam ring-opening/recyclization reaction of N-aryl-3-spirocyclic-β-lactams, made by the one-pot cyclization reaction of acetoacetanilides, has been achieved for the first time using a Lewis-Brønsted acids combined superacid catalyst system, thus providing an efficient entry to 3-spirocyclicquinolin-4(1H)-ones. A mechanism involving superacid-catalysis was proposed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
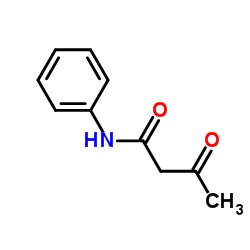 |
3-Oxo-N-phenylbutanamide
CAS:102-01-2 |
C10H11NO2 |
Related Articles:
More...
|
Kinetics and mechanisms of reactions between H2O2 and copper...
2015-09-28 [Dalton Trans. 44 , 16045-51, (2015)] |
|
Acetoacetanilides as masked isocyanates: facile and efficien...
2010-10-01 [Org. Lett. 12 , 4220-4223, (2010)] |
|
Massive screening yields novel and selectiveTrypanosoma cruz...
2010-01-01 [Eur. J. Med. Chem. 45 , 5767-72, (2010)] |
|
FeCl3⋅6H2O-catalyzed intermolecular-cascade cyclization of a...
2012-02-13 [Chemistry 18(7) , 1905-9, (2012)] |
|
Spectroscopic, and thermal studies of some new binuclear tra...
2007-11-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 68(3) , 918-26, (2007)] |