5-Aminouracil
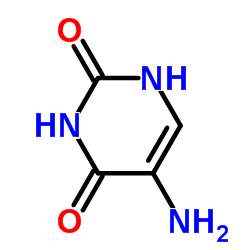
5-Aminouracil structure
|
Common Name | 5-Aminouracil | ||
|---|---|---|---|---|
| CAS Number | 932-52-5 | Molecular Weight | 127.101 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 580.4±53.0 °C at 760 mmHg | |
| Molecular Formula | C4H5N3O2 | Melting Point | >300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 304.8±30.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Effect of amino substitution on the excited state dynamics of uracil.
Photochem. Photobiol. Sci. 7(7) , 765-8, (2008) The excited state deactivation of two amino-substituted uracils, 5-aminouracil (5AU) and 6-aminouracil (6AU) in aqueous solution was studied by femtosecond fluorescence upconversion. The fluorescence of 6AU decays as fast as that of uracil with a unique time ... |
|
|
The peculiar spectral properties of amino-substituted uracils: a combined theoretical and experimental study.
J. Phys. Chem. B 114(39) , 12708-19, (2010) A detailed experimental and computational study of the absorption and fluorescence spectra of 5-aminouracil (5 AU) and 6-aminouracil (6 AU) in aqueous solution is reported. The lowest energy band of the steady-state absorption spectra of 5 AU is considerably ... |
|
|
Recovery from the 5-AU induced blockage in interphase: evidence for differential recovery.
Cytologia 47(3-4) , 545-53, (1982)
|
|
|
G2 checkpoint targets late replicating DNA.
Biol. Cell 95(8) , 521-6, (2003) In the multinucleate cells induced in Allium cepa L. meristems, the nuclei surrounded by the largest cytoplasm environment complete replication earlier (advanced nuclei), but have a longer G2, than the others (delayed nuclei). Thus, all nuclei break down the ... |
|
|
Synthesis of new pyrazolo[1,5-a]pyrimidine derivative using 5-aminouracil and ketene dithioacetal.
Arch. Pharm. Res. 22(6) , 571-4, (1999) Novel 3-cyano-2-(3-uracilyl-5-amino) pyrazolo [1,5-a]pyrimidine has been synthesized using 5-aminouracil as a starting material. |
|
|
DNA injury induced by 5-aminouracil and caffeine in G2 checkpoints path of higher plant cells.
Biocell 29(2) , 169-76, (2005) This work evaluated the qualitative and quantitative cellular changes induced by treatment with 5-aminouracil (5-AU) and a combination of 5-AU and caffeine in plant cells in relation to DNA damage, repaired damage, and residual damage. As biological material,... |
|
|
Induction of premature mitosis in S-blocked onion cells.
Cell Biol. Int. 22(11-12) , 867-74, (1998) Onion root-tip cells were blocked at S-phase by treating them with 5-aminouracil (5AU). These cells were then further treated with caffeine/2-aminopurine (Caf/2AP) or a combination of both in the presence of 5AU. These tyrosine kinase inhibitors were able to ... |
|
|
Novel acyclic amide-conjugated nucleosides and their analogues.
Nucleosides Nucleotides Nucleic Acids 28 , 103-117, (2009) An effective one-step synthesis of new amide-conjugated nucleosides and their analogues, in the presence of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) as the condensing agent, is presented. Substrate subunits carrying carboxyl... |
|
|
The action of 5-amino uracil on log growth and division-synchronized Tetrahymena.
Cell Tissue Kinet. 16(3) , 285-301, (1983) The influence of 5-amino uracil (5-AU) was investigated on the cell cycle of log growth and division-synchronized Tetrahymena pyriformis GL. The division index of log growth phase Tetrahymena was suppressed by 50% after 40 min in 8 mM 5-AU. Cells division-syn... |
|
|
[Antidepressant effect and mechanism of action of a novel aminouracil derivative].
Eksp. Klin. Farmakol. 74(2) , 16-8, (2011) The psychotropic activity of a series of new aminouracil derivatives was studied on the widely applied models of pharmacological screening in rats. The most expressed antidepressant effect at the stage of primary pharmacological screening was been revealed fo... |