| Structure | Name/CAS No. | Articles |
|---|---|---|
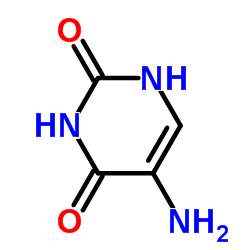 |
5-Aminouracil
CAS:932-52-5 |
|
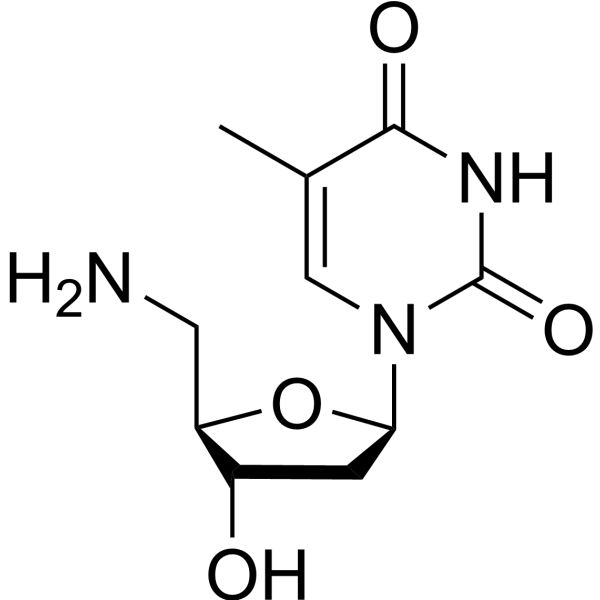 |
Thymidine,5'-amino-5'-deoxy
CAS:25152-20-9 |