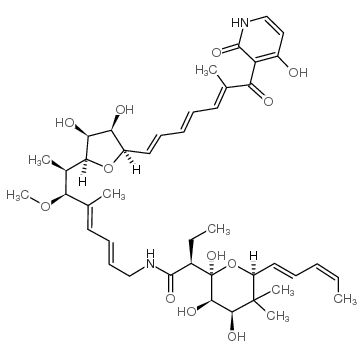
Kirromycin
Kirromycin structure
|
Common Name | Kirromycin | ||
|---|---|---|---|---|
| CAS Number | 50935-71-2 | Molecular Weight | 796.94300 | |
| Density | 1.279g/cm3 | Boiling Point | 936ºC at 760 mmHg | |
| Molecular Formula | C43H60N2O12 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 519.9ºC | |
|
A signal relay between ribosomal protein S12 and elongation factor EF-Tu during decoding of mRNA.
RNA 15(2) , 208-14, (2009) Codon recognition by aminoacyl-tRNA on the ribosome triggers a process leading to GTP hydrolysis by elongation factor Tu (EF-Tu) and release of aminoacyl-tRNA into the A site of the ribosome. The nature of this signal is largely unknown. Here, we present gene... |
|
|
The phosphopantetheinyl transferase KirP activates the ACP and PCP domains of the kirromycin NRPS/PKS of Streptomyces collinus Tü 365.
FEMS Microbiol. Lett. 319(1) , 26-33, (2011) The main steps in the biosynthesis of complex secondary metabolites such as the antibiotic kirromycin are catalyzed by modular polyketide synthases (PKS) and/or nonribosomal peptide synthetases (NRPS). During antibiotic assembly, the biosynthetic intermediate... |
|
|
Thermodynamic properties of nucleotide-free EF-Tu from Thermus thermophilus in the presence of low-molecular weight effectors of its GTPase activity.
Biochim. Biophys. Acta 1597(1) , 22-7, (2002) The thermal transition of elongation factor EF-Tu from Thermus thermophilus in the presence of low-molecular weight effectors was studied by differential scanning calorimetry. The effectors of GTPase activity used were the antibiotic kirromycin and the cation... |
|
|
Inhibitory mechanisms of antibiotics targeting elongation factor Tu.
Curr. Protein Pept. Sci. 3(1) , 121-31, (2002) Since the pioneering discovery of the inhibitory effects of kirromycin on bacterial elongation factor Tu (EF-Tu) more than 25 years ago [1], a great wealth of biological data has accumulated concerning protein biosynthesis inhibitors specific for EF-Tu. With ... |
|
|
G13A substitution affects the biochemical and physical properties of the elongation factor 1 alpha. A reduced intrinsic GTPase activity is partially restored by kirromycin.
Biochemistry 41(2) , 628-33, (2002) The G13A substitution in the G13XXXXGK[T,S] consensus sequence of the elongation factor 1 alpha from the archaeon Sulfolobus solfataricus (SsEF-1 alpha) was introduced in order to study the reasons for selective differences found in the homologous consensus e... |
|
|
The kirromycin gene cluster of Streptomyces collinus Tü 365 codes for an aspartate-alpha-decarboxylase, KirD, which is involved in the biosynthesis of the precursor beta-alanine.
J. Antibiot. 62(8) , 465-8, (2009)
|
|
|
Valine 114 replacements in archaeal elongation factor 1 alpha enhanced its ability to interact with aminoacyl-tRNA and kirromycin.
Biochemistry 41(49) , 14482-8, (2002) Valine 114 in the D(109)AAILVVA sequence of elongation factor 1alpha from the archaeon Sulfolobus solfataricus (SsEF-1alpha) was substituted with an acidic (V114E), basic (V114K), or cavity-forming (V114A) residue, and the effects on the biochemical propertie... |
|
|
Changes in ribosome function induced by protein kinase associated with ribosomes of Streptomyces collinus producing kirromycin.
Biochem. Biophys. Res. Commun. 289(2) , 434-43, (2001) Protein kinase associated with ribosomes of streptomycetes phosphorylates 11 ribosomal proteins. Phosphorylation activity of protein kinase reaches its maximum at the end of exponential phase of growth. When (32)P-labeled cells from the end of exponential pha... |
|
|
Isolation and characterization of dcw cluster from Streptomyces collinus producing kirromycin.
Biochem. Biophys. Res. Commun. 268(2) , 282-8, (2000) A 4.5-kb BamHI fragment of chromosomal DNA of Streptomyces collinus containing gene ftsZ was cloned and sequenced. Upstream of ftsZ are localized genes ftsQ, murG, and ftsW, and downstream is yfiH. Gene ftsA is not adjacent to ftsZ or other genes of the clone... |
|
|
Structural biology. A glimpse into tmRNA-mediated ribosome rescue.
Science 300(5616) , 72-3, (2003)
|